Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of a Self-Purification and Self-Assembly Coenzyme Regeneration System for the Synthesis of Chiral Drug Intermediates
ACS Omega ( IF 3.7 ) Pub Date : 2021-01-12 , DOI: 10.1021/acsomega.0c04668 Wei Jiang 1 , Wanru Zeng 1
ACS Omega ( IF 3.7 ) Pub Date : 2021-01-12 , DOI: 10.1021/acsomega.0c04668 Wei Jiang 1 , Wanru Zeng 1
Affiliation
|
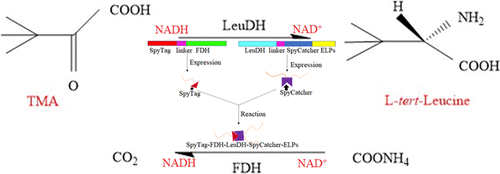
As one of the important research contents of synthetic biology, the construction of a regulatory system exhibits great potential in the synthesis of high value-added chemicals such as drug intermediates. In this work, a self-assembly coenzyme regeneration system, leucine dehydrogenase (LeuDH)–formate dehydrogenase (FDH) protein co-assembly system, was constructed by using the polypeptide, SpyTag/SpyCatcher. Then, it was demonstrated that the nonchromatographic inverse transition cycling purification method could purify intracellular coupling proteins and extracellular coupling proteins well. The conversion rate of the pure LeuDH–FDH protein assembly (FR-LR) was shown to be 1.6-fold and 32.3-fold higher than that of the free LeuDH–FDH system (LeuDH + FDH) and free LeuDH, respectively. This work has paved a new way of constructing a protein self-assembly system and engineering self-purification coenzyme regeneration system for the synthesis of chiral amino acids or chiral α-hydroxy acids.
中文翻译:

用于手性药物中间体合成的自纯化自组装辅酶再生系统的构建
作为合成生物学重要的研究内容之一,调控体系的构建在合成高附加值化学品如药物中间体方面显示出巨大的潜力。在这项工作中,使用多肽SpyTag / SpyCatcher构建了自组装辅酶再生系统,即亮氨酸脱氢酶(LeuDH)-甲酸盐脱氢酶(FDH)蛋白质共组装系统。证明了非色谱逆过渡循环纯化方法能够很好地纯化细胞内偶联蛋白和细胞外偶联蛋白。事实证明,纯LeuDH–FDH蛋白装配体(FR-LR)的转化率分别比游离LeuDH–FDH系统(LeuDH + FDH)和游离LeuDH高1.6倍和32.3倍。
更新日期:2021-01-26
中文翻译:

用于手性药物中间体合成的自纯化自组装辅酶再生系统的构建
作为合成生物学重要的研究内容之一,调控体系的构建在合成高附加值化学品如药物中间体方面显示出巨大的潜力。在这项工作中,使用多肽SpyTag / SpyCatcher构建了自组装辅酶再生系统,即亮氨酸脱氢酶(LeuDH)-甲酸盐脱氢酶(FDH)蛋白质共组装系统。证明了非色谱逆过渡循环纯化方法能够很好地纯化细胞内偶联蛋白和细胞外偶联蛋白。事实证明,纯LeuDH–FDH蛋白装配体(FR-LR)的转化率分别比游离LeuDH–FDH系统(LeuDH + FDH)和游离LeuDH高1.6倍和32.3倍。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号