当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of W-O Bonding on Gas Sensitivity of Nanocrystalline Bi2WO6 and WO3
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jallcom.2020.158159 Artem Marikutsa , Lili Yang , Alexey N. Kuznetsov , Marina Rumyantseva , Alexander Gaskov
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.jallcom.2020.158159 Artem Marikutsa , Lili Yang , Alexey N. Kuznetsov , Marina Rumyantseva , Alexander Gaskov
|
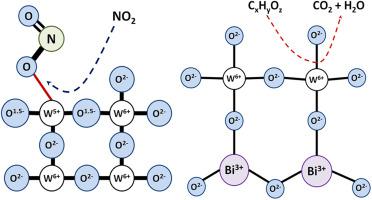
ABSTRACT It is known that adsorptive capacity and surface reactivity of metal oxides depends on chemical composition, which influences the characteristics of metal-oxygen bonds, e.g. degree of covalency, effective atomic charges, bond energy and bond length. Tungsten oxide and bismuth tungstate have perovskite-related structures and close semiconductor properties, but different chemical composition. The presence of bismuth in Bi2WO6 results in distinct W-O bond length and bond energy, respective to WO3, which may be one of the factors controlling the surface reactivity, as well as the occurrence of bismuth-related surface sites. Tungsten oxide is a renowned material for heterogeneous catalysts, photocatalysts, and sensors. Bismuth tungstate has been extensively studied as a photocatalytic material, and an interest in sensor applications of this compound emerged recently. Yet, there is a lack of comparative and systematic studies of the electronic structure and sensing properties of tungsten oxide and bismuth tungstate. Such a study would be promising for the elucidation of the role of W-O bonds in controlling the sensing behavior to different analyte gases. In this work, the comparative study of electronic and sensing properties of Bi2WO6 and WO3 was performed. Band structures, charge distribution and metal-oxygen bonds energies were calculated by first principles quantum chemical approach. Nanocrystalline Bi2WO6, WO3 and composite Bi2WO6+WO3 were synthesized. Bismuth tungstate showed an improved sensitivity to volatile organic compounds (ethanol, formaldehyde, acetone, benzene) and poor sensitivity to nitrogen dioxide, in contrast to WO3. Based on the experimental and computational data, the effects of W-O bond energy and charge distribution on the sensitivity to target gases of bismuth tungstate and tungsten oxide were rationalized.
中文翻译:

WO键对纳米晶Bi2WO6和WO3气敏性的影响
摘要众所周知,金属氧化物的吸附能力和表面反应性取决于化学成分,化学成分会影响金属-氧键的特性,例如共价程度、有效原子电荷、键能和键长。氧化钨和钨酸铋具有钙钛矿相关的结构和接近的半导体性质,但化学成分不同。Bi2WO6 中铋的存在导致与 WO3 不同的 WO 键长和键能,这可能是控制表面反应性的因素之一,以及与铋相关的表面位点的出现。氧化钨是一种著名的多相催化剂、光催化剂和传感器材料。钨酸铋作为光催化材料已被广泛研究,最近出现了对该化合物的传感器应用的兴趣。然而,缺乏对氧化钨和钨酸铋的电子结构和传感特性的比较和系统的研究。这样的研究有望阐明 WO 键在控制对不同分析物气体的传感行为方面的作用。在这项工作中,对 Bi2WO6 和 WO3 的电子和传感特性进行了比较研究。通过第一原理量子化学方法计算能带结构、电荷分布和金属-氧键能。合成了纳米晶Bi2WO6、WO3和复合Bi2WO6+WO3。与 WO3 相比,钨酸铋对挥发性有机化合物(乙醇、甲醛、丙酮、苯)的敏感性提高,而对二氧化氮的敏感性较差。
更新日期:2021-03-01
中文翻译:

WO键对纳米晶Bi2WO6和WO3气敏性的影响
摘要众所周知,金属氧化物的吸附能力和表面反应性取决于化学成分,化学成分会影响金属-氧键的特性,例如共价程度、有效原子电荷、键能和键长。氧化钨和钨酸铋具有钙钛矿相关的结构和接近的半导体性质,但化学成分不同。Bi2WO6 中铋的存在导致与 WO3 不同的 WO 键长和键能,这可能是控制表面反应性的因素之一,以及与铋相关的表面位点的出现。氧化钨是一种著名的多相催化剂、光催化剂和传感器材料。钨酸铋作为光催化材料已被广泛研究,最近出现了对该化合物的传感器应用的兴趣。然而,缺乏对氧化钨和钨酸铋的电子结构和传感特性的比较和系统的研究。这样的研究有望阐明 WO 键在控制对不同分析物气体的传感行为方面的作用。在这项工作中,对 Bi2WO6 和 WO3 的电子和传感特性进行了比较研究。通过第一原理量子化学方法计算能带结构、电荷分布和金属-氧键能。合成了纳米晶Bi2WO6、WO3和复合Bi2WO6+WO3。与 WO3 相比,钨酸铋对挥发性有机化合物(乙醇、甲醛、丙酮、苯)的敏感性提高,而对二氧化氮的敏感性较差。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号