当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical Rearrangement of Aryl/Alkylidene Malononitriles via Aza Michael Addition/Decynoformylation/Addition Sequence: An Access to α-Aminonitriles and α-Aminoamides
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.joc.0c01358 Shubhangi P. Bhoite 1, 2 , Ajay H. Bansode 1, 2 , Gurunath Suryavanshi 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-11-04 , DOI: 10.1021/acs.joc.0c01358 Shubhangi P. Bhoite 1, 2 , Ajay H. Bansode 1, 2 , Gurunath Suryavanshi 1, 2
Affiliation
|
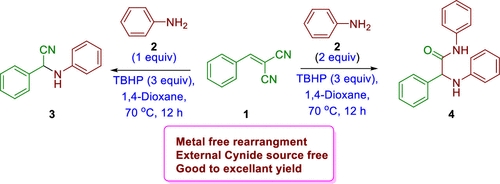
An efficient, safe, and environmentally friendly tertiary butyl hydrogen peroxide (TBHP)-mediated rearrangement of aryl/alkylidene malononitrile with anilines has been developed with in situ generation of HCN as the cyanide source for the synthesis of substituted α-aminonitriles and α-aminoamide. A diverse set of α-aminonitriles and α-aminoamides was efficiently synthesized in good to excellent yields. This method features a broad substrate scope and good functional group tolerance, and the in situ-generated HCN bypasses the use of an external cyanide source.
中文翻译:

通过Aza Michael加成/癸基甲酰化/加成序列对芳基/亚烷基丙二腈进行自由基重排:获得α-氨基腈和α-氨基酰胺
已经开发出一种高效,安全,环保的叔丁基过氧化氢(TBHP)介导的芳基/亚烷基丙二腈与苯胺的重排反应,并原位生成HCN作为氰化物来源,用于合成取代的α-氨基腈和α-氨基酰胺。有效地合成了多种多样的α-氨基腈和α-氨基酰胺,收率很高。该方法具有广泛的底物范围和良好的官能团耐受性,并且原位生成的HCN无需使用外部氰化物源。
更新日期:2020-12-04
中文翻译:

通过Aza Michael加成/癸基甲酰化/加成序列对芳基/亚烷基丙二腈进行自由基重排:获得α-氨基腈和α-氨基酰胺
已经开发出一种高效,安全,环保的叔丁基过氧化氢(TBHP)介导的芳基/亚烷基丙二腈与苯胺的重排反应,并原位生成HCN作为氰化物来源,用于合成取代的α-氨基腈和α-氨基酰胺。有效地合成了多种多样的α-氨基腈和α-氨基酰胺,收率很高。该方法具有广泛的底物范围和良好的官能团耐受性,并且原位生成的HCN无需使用外部氰化物源。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号