Polymer ( IF 4.1 ) Pub Date : 2020-11-02 , DOI: 10.1016/j.polymer.2020.123165 Balázs Pásztói , Tobias M. Trötschler , Ákos Szabó , Benjamin Kerscher , Heikki Tenhu , Rolf Mülhaupt , Béla Iván
|
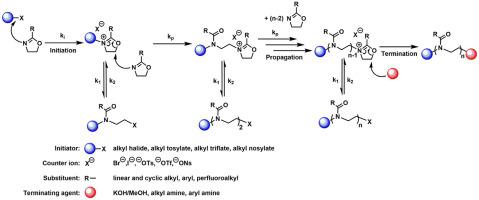
Cationic ring-opening polymerization (CROP) of 2-ethyl-2-oxazoline (EtOx) was systematically investigated in benzotrifluoride (BTF), which is considered as an environmentally less harmful solvent than many conventional reaction media. Simultaneously, polymerizations in conventional solvents, such as acetonitrile, N,N-dimethylacetamide and toluene, were also carried out for comparison in the 80–100 °C temperature range. Kinetic experiments revealed that the monomer consumption occurs by first order kinetics and the number average molecular weights linearly increase in line with the theoretical molecular weight as a function of monomer conversion. These findings indicate that the polymerization takes place by quasiliving CROP in all the investigated solvents, including BTF as well, resulting in PEtOx with prederminded molecular weights and polydispersities of 1.3–15. The highest polymerization rates were obtained in BTF, resulting in high conversions in short reaction times at 100 °C reaction temperature. The Arrhenius parameters of the polymerization of EtOx in BTF indicates relatively high activation energy in comparison with other applied solvents, however, a compensation effect between the activation energies and frequency factor is observed for such polymerization in a variety of solvents. Our findings are expected to enable the convenient synthesis of polyoxazolines and polyoxazoline-based well-defined polymer architectures in BTF, an environmentally advantageous alternative solvent to harmful polymerization media, with high polymerization rates in short reaction times without the need for any special conditions or equipment.
中文翻译:

2-乙基-2-恶唑啉在苯并三氟化物中的拟活化阳离子开环聚合反应,作为替代反应介质
在苯并三氟化物(BTF)中系统地研究了2-乙基-2-恶唑啉(EtOx)的阳离子开环聚合(CROP),该物质被认为比许多常规反应介质对环境的危害小。同时,在80–100°C的温度范围内,还进行了常规溶剂(如乙腈,N,N-二甲基乙酰胺和甲苯)中的聚合反应进行比较。动力学实验表明,单体消耗是通过一级动力学发生的,并且数均分子量与理论分子量成线性关系,是单体转化的函数。这些发现表明,聚合反应是通过在所有研究的溶剂(包括BTF)中将CROP准化来实现的,导致PEtOx具有预先确定的分子量和多分散性1.3–15。在BTF中获得了最高的聚合速率,从而在较短的反应时间内在100°C的反应温度下实现了高转化率。与其他应用的溶剂相比,EtOx在BTF中聚合的Arrhenius参数显示出较高的活化能,但是,在各种溶剂中,这种聚合反应均观察到活化能和频率因子之间的补偿作用。我们的发现有望在BTF(一种对环境有害的有害聚合介质的替代溶剂)中方便合成聚恶唑啉和基于聚恶唑啉的明确定义的聚合物结构,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号