当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Insights into the Electrochemical Mechanism of Rechargeable Calcium/Sulfur Batteries
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.chemmater.0c02074 Antonio Scafuri 1, 2, 3, 4 , Romain Berthelot 2, 3, 5 , Klemen Pirnat 1 , Alen Vizintin 1 , Jan Bitenc 1 , Giuliana Aquilanti 6 , Dominique Foix 5, 7 , Rémi Dedryvère 3, 5, 7 , Iztok Arčon 8, 9 , Robert Dominko 1, 3, 4 , Lorenzo Stievano 2, 3, 5
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.chemmater.0c02074 Antonio Scafuri 1, 2, 3, 4 , Romain Berthelot 2, 3, 5 , Klemen Pirnat 1 , Alen Vizintin 1 , Jan Bitenc 1 , Giuliana Aquilanti 6 , Dominique Foix 5, 7 , Rémi Dedryvère 3, 5, 7 , Iztok Arčon 8, 9 , Robert Dominko 1, 3, 4 , Lorenzo Stievano 2, 3, 5
Affiliation
|
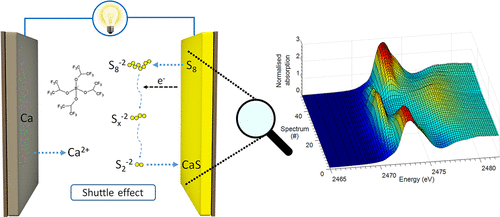
Calcium batteries represent a promising alternative to lithium metal systems. The combination of the low redox potential and low cost and the energy-dense calcium anode (2073 mAh/cm3, similar to 2044 mAh/cm3 for Li) with appropriate low-cost cathode materials such as sulfur could produce a game-changing technology in several fields of applications. In this work, we present the reversible activity of a proof-of-concept Ca/S battery at room temperature, characterized by a surprising medium-term cycling stability with low polarization, promoted by the use of a simple positive electrode made of sulfur supported on an activated carbon cloth scaffold, and a state-of-the-art fluorinated alkoxyborate-based electrolyte. Insights into the electrochemical mechanism governing the chemistry of the Ca/S system were obtained for the first time by combining X-ray photoelectron spectroscopy and X-ray absorption spectroscopy. The mechanism implies the formation of different types of soluble polysulfide species during both charge and discharge at room temperature, and the formation of solid CaS at the end of discharge. The reversible electrochemical activity is proven by the reformation of elemental sulfur at the end of the following charge. These promising results open the way to the comprehension of emerging Ca/S systems, which may represent a valid alternative to Mg/S and Li/S batteries.
中文翻译:

光谱学对可充电钙/硫电池电化学机理的认识
钙电池是锂金属系统的有前途的替代品。低氧化还原电势和低成本与高能量钙阳极(2073 mAh / cm 3,类似于2044 mAh / cm 3的组合)对于Li),使用适当的低成本阴极材料(例如硫)可以在多个应用领域产生改变游戏规则的技术。在这项工作中,我们展示了概念验证型Ca / S电池在室温下的可逆活性,其特征在于令人惊讶的中期循环稳定性和低极化性,这是通过使用由硫磺制成的简单正电极促进的放在活性炭布支架上,以及最先进的氟化烷氧基硼酸酯基电解质。通过结合X射线光电子能谱和X射线吸收能谱首次获得了对控制Ca / S系统化学作用的电化学机理的见解。该机理暗示在室温下充放电过程中会形成不同类型的可溶性多硫化物,并在放电结束时形成固态CaS。可逆的电化学活性通过在后续加料结束时重整元素硫来证明。这些有希望的结果为理解新兴的Ca / S系统开辟了道路,这可能是Mg / S和Li / S电池的有效替代品。
更新日期:2020-10-13
中文翻译:

光谱学对可充电钙/硫电池电化学机理的认识
钙电池是锂金属系统的有前途的替代品。低氧化还原电势和低成本与高能量钙阳极(2073 mAh / cm 3,类似于2044 mAh / cm 3的组合)对于Li),使用适当的低成本阴极材料(例如硫)可以在多个应用领域产生改变游戏规则的技术。在这项工作中,我们展示了概念验证型Ca / S电池在室温下的可逆活性,其特征在于令人惊讶的中期循环稳定性和低极化性,这是通过使用由硫磺制成的简单正电极促进的放在活性炭布支架上,以及最先进的氟化烷氧基硼酸酯基电解质。通过结合X射线光电子能谱和X射线吸收能谱首次获得了对控制Ca / S系统化学作用的电化学机理的见解。该机理暗示在室温下充放电过程中会形成不同类型的可溶性多硫化物,并在放电结束时形成固态CaS。可逆的电化学活性通过在后续加料结束时重整元素硫来证明。这些有希望的结果为理解新兴的Ca / S系统开辟了道路,这可能是Mg / S和Li / S电池的有效替代品。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号