当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brønsted Acid-Catalyzed Cyanotritylation of Aldehydes by Trityl Isocyanide
Organic Letters ( IF 4.9 ) Pub Date : 2016-07-12 00:00:00 , DOI: 10.1021/acs.orglett.6b01481 Răzvan C. Cioc 1 , Peter Schuckman 1 , Hans D. Preschel 1 , Tjøstil Vlaar 1 , Eelco Ruijter 1 , Romano V. A. Orru 1
Organic Letters ( IF 4.9 ) Pub Date : 2016-07-12 00:00:00 , DOI: 10.1021/acs.orglett.6b01481 Răzvan C. Cioc 1 , Peter Schuckman 1 , Hans D. Preschel 1 , Tjøstil Vlaar 1 , Eelco Ruijter 1 , Romano V. A. Orru 1
Affiliation
|
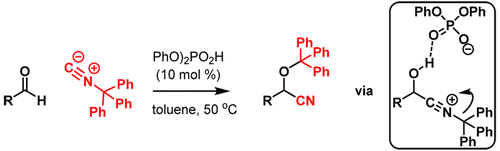
Cyanohydrins are versatile intermediates toward valuable organic compounds like α-hydroxy carboxylic acids, α-amino acids, and β-amino alcohols. Numerous protocols are available for synthesis of (O-protected) cyanohydrins, but all procedures invariably rely on the use of toxic cyanide sources. A novel cyanide-free synthesis of O-trityl protected cyanohydrins via a catalytic Passerini-type reaction involving aldehydes and trityl isocyanide is reported. The feasibility of a catalytic asymmetric reaction is demonstrated using chiral phosphoric acid catalysis.
中文翻译:

三苯甲基异氰酸酯对布朗斯台德酸催化醛的氰基化反应
氰醇是通用的中间体,可用于有价值的有机化合物,例如α-羟基羧酸,α-氨基酸和β-氨基醇。合成(O-保护的)氰醇的方法很多,但是所有方法总是依赖使用有毒的氰化物源。据报道,通过涉及醛和三苯甲基异氰化物的Passerini型催化反应,新型的无氰基保护的O-三苯甲基保护的氰醇合成。使用手性磷酸催化证明了催化不对称反应的可行性。
更新日期:2016-07-12
中文翻译:

三苯甲基异氰酸酯对布朗斯台德酸催化醛的氰基化反应
氰醇是通用的中间体,可用于有价值的有机化合物,例如α-羟基羧酸,α-氨基酸和β-氨基醇。合成(O-保护的)氰醇的方法很多,但是所有方法总是依赖使用有毒的氰化物源。据报道,通过涉及醛和三苯甲基异氰化物的Passerini型催化反应,新型的无氰基保护的O-三苯甲基保护的氰醇合成。使用手性磷酸催化证明了催化不对称反应的可行性。





























 京公网安备 11010802027423号
京公网安备 11010802027423号