当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling the Mechanism of CO2/Cyclohexene Oxide Copolymerization Catalyzed by Chiral Zinc β-Diiminates: Factors Affecting Reactivity and Isotacticity
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-15 , DOI: 10.1021/acscatal.0c02299 Huiling Shao 1 , Yernaidu Reddi 1 , Christopher J. Cramer 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-07-15 , DOI: 10.1021/acscatal.0c02299 Huiling Shao 1 , Yernaidu Reddi 1 , Christopher J. Cramer 1
Affiliation
|
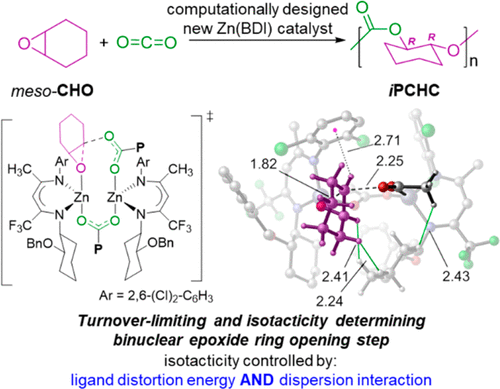
Copolymerization of CO2 and cyclohexene oxide (CHO) upcycles CO2 into the value-added, chemically recyclable, thermoplastic poly(cyclohexene carbonate) (PCHC). Using density functional theory, the Zn-catalyzed copolymerization mechanism has been characterized with a particular focus on the effects of chiral β-diiminate (BDI) ligands as they influence the reactivity and enantioselectivity in the epoxide ring-opening step, where the latter is required for isotacticity. Theory indicates that both mono- and binuclear forms of the catalyst are involved along the reaction path, with the turnover-limiting step being ring-opening of the epoxide mediated by a binuclear catalyst. Subsequent CO2 insertion is predicted to be kinetically facile and preferentially mediated by a mononuclear catalyst. The predicted preference for epoxide opening to give R,R-stereocenters in the copolymer when N-(4-(((1S,2S)-2-(benzyloxy)cyclohexyl)amino)-5,5,5-trifluoropent-3-en-2-ylidene)-2,6-dimethylaniline is used as the BDI ligand agrees with the experiment and is attributed to differential ligand distortions associated with key non-bonded interactions in the competing transition-state structures. Further analysis predicts that 2,6-dichloro and dibromo substitutions of the BDI ligand N-aryl group(s) should result in increased rates and enantioselectivities for copolymerization.
中文翻译:

β-二氨基手性锌催化CO 2 /环己烯氧化物共聚机理的建模:影响反应性和全同立构规整度的因素
CO 2和环氧化环氧乙烷(CHO)的共聚将CO 2循环到增值的,可化学回收的热塑性聚碳酸环己酯(PCHC)中。使用密度泛函理论,表征了锌催化的共聚机理,特别关注手性β-二氨基(BDI)配体的影响,因为它们会影响环氧化物开环步骤中的反应性和对映选择性,而后者是必需的等规度。理论表明,单核形式和双核形式的催化剂都沿着反应路径参与,其中周转限制步骤是由双核催化剂介导的环氧化物的开环。随后的CO 2预测插入是动力学上容易的并且优选地由单核催化剂介导。N-(4-((((1 S,2 S)-2-(苄氧基)环己基)氨基)-5,5,5-三氟戊-时,环氧化物开环在共聚物中产生R,R-立体中心的预测偏好3-烯-2-亚丙基)-2,6-二甲基苯胺被用作BDI配体与实验相符,并归因于与竞争过渡态结构中关键非键合相互作用相关的不同配体扭曲。进一步的分析预测,BDI配体N-芳基的2,6-二氯和二溴取代应导致共聚反应的速率和对映选择性增加。
更新日期:2020-08-08
中文翻译:

β-二氨基手性锌催化CO 2 /环己烯氧化物共聚机理的建模:影响反应性和全同立构规整度的因素
CO 2和环氧化环氧乙烷(CHO)的共聚将CO 2循环到增值的,可化学回收的热塑性聚碳酸环己酯(PCHC)中。使用密度泛函理论,表征了锌催化的共聚机理,特别关注手性β-二氨基(BDI)配体的影响,因为它们会影响环氧化物开环步骤中的反应性和对映选择性,而后者是必需的等规度。理论表明,单核形式和双核形式的催化剂都沿着反应路径参与,其中周转限制步骤是由双核催化剂介导的环氧化物的开环。随后的CO 2预测插入是动力学上容易的并且优选地由单核催化剂介导。N-(4-((((1 S,2 S)-2-(苄氧基)环己基)氨基)-5,5,5-三氟戊-时,环氧化物开环在共聚物中产生R,R-立体中心的预测偏好3-烯-2-亚丙基)-2,6-二甲基苯胺被用作BDI配体与实验相符,并归因于与竞争过渡态结构中关键非键合相互作用相关的不同配体扭曲。进一步的分析预测,BDI配体N-芳基的2,6-二氯和二溴取代应导致共聚反应的速率和对映选择性增加。





























 京公网安备 11010802027423号
京公网安备 11010802027423号