当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function.
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2020-07-01 , DOI: 10.1124/pr.119.018531 Katie Leach 1 , Fadil M Hannan 2 , Tracy M Josephs 2 , Andrew N Keller 2 , Thor C Møller 2 , Donald T Ward 2 , Enikö Kallay 2 , Rebecca S Mason 2 , Rajesh V Thakker 2 , Daniela Riccardi 2 , Arthur D Conigrave 2 , Hans Bräuner-Osborne 1
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2020-07-01 , DOI: 10.1124/pr.119.018531 Katie Leach 1 , Fadil M Hannan 2 , Tracy M Josephs 2 , Andrew N Keller 2 , Thor C Møller 2 , Donald T Ward 2 , Enikö Kallay 2 , Rebecca S Mason 2 , Rajesh V Thakker 2 , Daniela Riccardi 2 , Arthur D Conigrave 2 , Hans Bräuner-Osborne 1
Affiliation
|
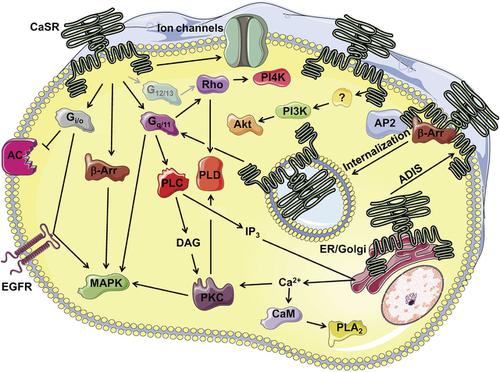
The calcium-sensing receptor (CaSR) is a class C G protein–coupled receptor that responds to multiple endogenous agonists and allosteric modulators, including divalent and trivalent cations, L-amino acids, γ-glutamyl peptides, polyamines, polycationic peptides, and protons. The CaSR plays a critical role in extracellular calcium (Ca2+o) homeostasis, as demonstrated by the many naturally occurring mutations in the CaSR or its signaling partners that cause Ca2+o homeostasis disorders. However, CaSR tissue expression in mammals is broad and includes tissues unrelated to Ca2+o homeostasis, in which it, for example, regulates the secretion of digestive hormones, airway constriction, cardiovascular effects, cellular differentiation, and proliferation. Thus, although the CaSR is targeted clinically by the positive allosteric modulators (PAMs) cinacalcet, evocalcet, and etelcalcetide in hyperparathyroidism, it is also a putative therapeutic target in diabetes, asthma, cardiovascular disease, and cancer. The CaSR is somewhat unique in possessing multiple ligand binding sites, including at least five putative sites for the “orthosteric” agonist Ca2+o, an allosteric site for endogenous L-amino acids, two further allosteric sites for small molecules and the peptide PAM, etelcalcetide, and additional sites for other cations and anions. The CaSR is promiscuous in its G protein–coupling preferences, and signals via Gq/11, Gi/o, potentially G12/13, and even Gs in some cell types. Not surprisingly, the CaSR is subject to biased agonism, in which distinct ligands preferentially stimulate a subset of the CaSR’s possible signaling responses, to the exclusion of others. The CaSR thus serves as a model receptor to study natural bias and allostery.
中文翻译:

国际基础与临床药理学联盟。 CVIII。钙敏感受体命名法、药理学和功能。
钙敏感受体 (CaSR) 是一类 CG 蛋白偶联受体,可响应多种内源性激动剂和变构调节剂,包括二价和三价阳离子、L-氨基酸、 γ-谷氨酰肽、多胺、聚阳离子肽和质子。 CaSR 在细胞外钙 (Ca 2+ o ) 稳态中发挥着关键作用,CaSR 或其信号伙伴中许多自然发生的突变导致 Ca 2+ o稳态紊乱就证明了这一点。然而,CaSR在哺乳动物中的组织表达范围很广,包括与Ca 2+ o稳态无关的组织,例如,它在其中调节消化激素的分泌、气道收缩、心血管效应、细胞分化和增殖。因此,尽管 CaSR 在临床上是甲状旁腺功能亢进症中正变构调节剂 (PAM) 西那卡塞、依沃卡塞和埃替卡塞肽的靶标,但它也是糖尿病、哮喘、心血管疾病和癌症的假定治疗靶点。 CaSR 的独特之处在于拥有多个配体结合位点,包括至少五个“正构”激动剂 Ca 2+ o的推定位点、一个内源性 L-氨基酸的变构位点、另外两个小分子和肽 PAM 的变构位点、etelcalcetide 以及其他阳离子和阴离子的附加位点。 CaSR 的 G 蛋白偶联偏好是混杂的,并且在某些细胞类型中通过 G q/11 、 G i/o 、潜在的 G 12/13甚至 G s发出信号。 毫不奇怪,CaSR 受到偏向激动作用,其中不同的配体优先刺激 CaSR 可能的信号反应的一部分,而排除其他配体。因此,CaSR 可作为研究自然偏向和变构的模型受体。
更新日期:2020-05-29
中文翻译:

国际基础与临床药理学联盟。 CVIII。钙敏感受体命名法、药理学和功能。
钙敏感受体 (CaSR) 是一类 CG 蛋白偶联受体,可响应多种内源性激动剂和变构调节剂,包括二价和三价阳离子、L-氨基酸、 γ-谷氨酰肽、多胺、聚阳离子肽和质子。 CaSR 在细胞外钙 (Ca 2+ o ) 稳态中发挥着关键作用,CaSR 或其信号伙伴中许多自然发生的突变导致 Ca 2+ o稳态紊乱就证明了这一点。然而,CaSR在哺乳动物中的组织表达范围很广,包括与Ca 2+ o稳态无关的组织,例如,它在其中调节消化激素的分泌、气道收缩、心血管效应、细胞分化和增殖。因此,尽管 CaSR 在临床上是甲状旁腺功能亢进症中正变构调节剂 (PAM) 西那卡塞、依沃卡塞和埃替卡塞肽的靶标,但它也是糖尿病、哮喘、心血管疾病和癌症的假定治疗靶点。 CaSR 的独特之处在于拥有多个配体结合位点,包括至少五个“正构”激动剂 Ca 2+ o的推定位点、一个内源性 L-氨基酸的变构位点、另外两个小分子和肽 PAM 的变构位点、etelcalcetide 以及其他阳离子和阴离子的附加位点。 CaSR 的 G 蛋白偶联偏好是混杂的,并且在某些细胞类型中通过 G q/11 、 G i/o 、潜在的 G 12/13甚至 G s发出信号。 毫不奇怪,CaSR 受到偏向激动作用,其中不同的配体优先刺激 CaSR 可能的信号反应的一部分,而排除其他配体。因此,CaSR 可作为研究自然偏向和变构的模型受体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号