当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Approach for Assembling Flavanones via a Cascade 1,4-Conjugate Addition/oxa-Michael Addition between Propargylamines with Water.
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.orglett.0c01357 Xinwei He 1, 2 , Mengqing Xie 1 , Ruxue Li 1 , Pui Ying Choy 2 , Qiang Tang 1 , Yongjia Shang 1 , Fuk Yee Kwong 2
Organic Letters ( IF 4.9 ) Pub Date : 2020-05-22 , DOI: 10.1021/acs.orglett.0c01357 Xinwei He 1, 2 , Mengqing Xie 1 , Ruxue Li 1 , Pui Ying Choy 2 , Qiang Tang 1 , Yongjia Shang 1 , Fuk Yee Kwong 2
Affiliation
|
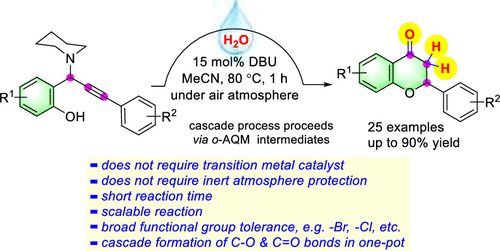
A DBU-catalyzed one-pot cascade reaction of propargylamines and water for the synthesis of flavanones has been developed. This process proceeds via a sequence of 1,4-conjugate addition of water to alkynyl o-quinone methide (o-AQM), followed by the alkyne–allene isomerization and subsequent intramolecular oxa-Michael addition. This strategy provides a convenient method for accessing a broad range of flavanones in good to excellent yields with good functional-group tolerance, in particular, the reactive halo functional groups.
中文翻译:

经由炔丙基胺与水之间的级联1,4-共轭加成/氧杂-迈克尔加成组装黄酮的有机催化方法。
已经开发了一种DBU催化的炔丙胺和水的一锅级联反应,用于合成黄烷酮。该过程是通过依次将水以1,4-共轭加成到炔基邻苯二甲酰甲基甲烷(o -AQM)上,然后进行炔烃-丙二烯异构化和随后的分子内氧杂-Michael加成反应。该策略提供了一种方便的方法,可以以良好的产率获得具有良好的官能团耐受性的宽泛的黄烷酮,特别是反应性卤素官能团。
更新日期:2020-05-22
中文翻译:

经由炔丙基胺与水之间的级联1,4-共轭加成/氧杂-迈克尔加成组装黄酮的有机催化方法。
已经开发了一种DBU催化的炔丙胺和水的一锅级联反应,用于合成黄烷酮。该过程是通过依次将水以1,4-共轭加成到炔基邻苯二甲酰甲基甲烷(o -AQM)上,然后进行炔烃-丙二烯异构化和随后的分子内氧杂-Michael加成反应。该策略提供了一种方便的方法,可以以良好的产率获得具有良好的官能团耐受性的宽泛的黄烷酮,特别是反应性卤素官能团。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号