当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting Regioselectivity in Radical C-H Functionalization of Heterocycles through Machine Learning.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-02 , DOI: 10.1002/anie.202000959 Xin Li 1 , Shuo-Qing Zhang 1 , Li-Cheng Xu 1 , Xin Hong 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-05-02 , DOI: 10.1002/anie.202000959 Xin Li 1 , Shuo-Qing Zhang 1 , Li-Cheng Xu 1 , Xin Hong 1
Affiliation
|
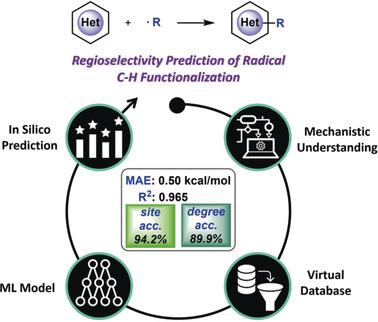
Radical C−H bond functionalization provides a versatile approach for elaborating heterocyclic compounds. The synthetic design of this transformation relies heavily on the knowledge of regioselectivity, while a quantified and efficient regioselectivity prediction approach is still elusive. Herein, we report the feasibility of using a machine learning model to predict the transition state barrier from the computed properties of isolated reactants. This enables rapid and reliable regioselectivity prediction for radical C−H bond functionalization of heterocycles. The Random Forest model with physical organic features achieved 94.2 % site accuracy and 89.9 % selectivity accuracy in the out‐of‐sample test set. The prediction performance was further validated by comparing the machine learning results with additional substituents, heteroarene scaffolds and experimental observations. This work revealed that the combination of mechanism‐based computational statistics and machine learning model can serve as a useful strategy for selectivity prediction of organic transformations.
中文翻译:

通过机器学习预测杂环自由基CH官能化中的区域选择性。
自由基CH键功能化为杂环化合物的制备提供了一种通用的方法。这种转化的综合设计在很大程度上依赖于区域选择性的知识,而量化和有效的区域选择性预测方法仍然难以捉摸。在这里,我们报告了使用机器学习模型从孤立的反应物的计算属性预测过渡态势垒的可行性。这使得能够快速可靠地预测杂环的C-H键官能化的区域选择性。具有物理有机特征的随机森林模型在样本外测试集中实现了94.2%的位点精度和89.9%的选择性精度。通过将机器学习结果与其他取代基进行比较,进一步验证了预测性能 杂芳烃支架和实验观察。这项工作表明,基于机制的计算统计数据和机器学习模型的结合可以作为有机转化的选择性预测的有用策略。
更新日期:2020-05-02
中文翻译:

通过机器学习预测杂环自由基CH官能化中的区域选择性。
自由基CH键功能化为杂环化合物的制备提供了一种通用的方法。这种转化的综合设计在很大程度上依赖于区域选择性的知识,而量化和有效的区域选择性预测方法仍然难以捉摸。在这里,我们报告了使用机器学习模型从孤立的反应物的计算属性预测过渡态势垒的可行性。这使得能够快速可靠地预测杂环的C-H键官能化的区域选择性。具有物理有机特征的随机森林模型在样本外测试集中实现了94.2%的位点精度和89.9%的选择性精度。通过将机器学习结果与其他取代基进行比较,进一步验证了预测性能 杂芳烃支架和实验观察。这项工作表明,基于机制的计算统计数据和机器学习模型的结合可以作为有机转化的选择性预测的有用策略。





























 京公网安备 11010802027423号
京公网安备 11010802027423号