当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Tetrazole- and Pyridine-Containing Itraconazole Analogs as Potent Angiogenesis Inhibitors.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-04-08 , DOI: 10.1021/acsmedchemlett.9b00438 Yingjun Li 1 , Kalyan Kumar Pasunooti 1 , Hanjing Peng 1 , Ruo-Jing Li 1 , Wei Q Shi 1 , Wukun Liu 1 , Zhiqiang Cheng 1 , Sarah A Head 1 , Jun O Liu 1, 2
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-04-08 , DOI: 10.1021/acsmedchemlett.9b00438 Yingjun Li 1 , Kalyan Kumar Pasunooti 1 , Hanjing Peng 1 , Ruo-Jing Li 1 , Wei Q Shi 1 , Wukun Liu 1 , Zhiqiang Cheng 1 , Sarah A Head 1 , Jun O Liu 1, 2
Affiliation
|
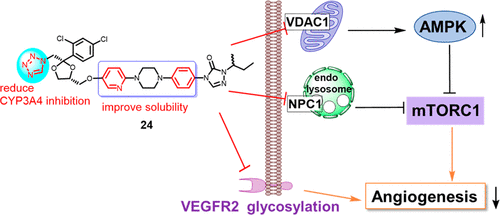
Itraconazole, a widely used antifungal drug, was found to possess antiangiogenic activity and is currently undergoing multiple clinical trials for the treatment of different types of cancer. However, it suffers from extremely low solubility and strong interactions with many drugs through inhibition of CYP3A4, limiting its potential as a new antiangiogenic and anticancer drug. To address these issues, a series of analogs in which the phenyl group is replaced with pyridine or fluorine-substituted benzene was synthesized. Among them the pyridine- and tetrazole-containing compound 24 has significantly improved solubility and reduced CYP3A4 inhibition compared to itraconazole. Similar to itraconazole, compound 24 inhibited the AMPK/mTOR signaling axis and the glycosylation of VEGFR2. It also induced cholesterol accumulation in the endolysosome and demonstrated binding to the sterol-sensing domain of NPC1 in a simulation study. These results suggested that compound 24 may serve as an attractive candidate for the development of a new generation of antiangiogenic drug.
中文翻译:

设计和合成含四唑和吡啶的伊曲康唑类似物作为强效血管生成抑制剂。
伊曲康唑是一种广泛使用的抗真菌药物,被发现具有抗血管生成活性,目前正接受多种临床试验来治疗不同类型的癌症。但是,它具有极低的溶解度和通过抑制CYP3A4与许多药物的强烈相互作用,限制了其作为新的抗血管生成和抗癌药物的潜力。为了解决这些问题,合成了一系列类似物,其中苯基被吡啶或氟取代的苯取代。其中,与伊曲康唑相比,含吡啶和四唑的化合物24的溶解度显着提高,并且对CYP3A4的抑制作用降低。与伊曲康唑相似,化合物24抑制AMPK / mTOR信号轴和VEGFR2的糖基化。在模拟研究中,它还诱导胆固醇在溶酶体中积累,并证明与NPC1的固醇感应域结合。这些结果表明,化合物24可以作为开发新一代抗血管生成药物的有吸引力的候选物。
更新日期:2020-04-08
中文翻译:

设计和合成含四唑和吡啶的伊曲康唑类似物作为强效血管生成抑制剂。
伊曲康唑是一种广泛使用的抗真菌药物,被发现具有抗血管生成活性,目前正接受多种临床试验来治疗不同类型的癌症。但是,它具有极低的溶解度和通过抑制CYP3A4与许多药物的强烈相互作用,限制了其作为新的抗血管生成和抗癌药物的潜力。为了解决这些问题,合成了一系列类似物,其中苯基被吡啶或氟取代的苯取代。其中,与伊曲康唑相比,含吡啶和四唑的化合物24的溶解度显着提高,并且对CYP3A4的抑制作用降低。与伊曲康唑相似,化合物24抑制AMPK / mTOR信号轴和VEGFR2的糖基化。在模拟研究中,它还诱导胆固醇在溶酶体中积累,并证明与NPC1的固醇感应域结合。这些结果表明,化合物24可以作为开发新一代抗血管生成药物的有吸引力的候选物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号