当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of the Oxygen Vacancies and Structural Order on the Oxygen Evolution Activity: A Case Study of SrMnO3−δ Featuring Four Different Structure Types
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.inorgchem.9b03774 Ram Krishna Hona 1 , Farshid Ramezanipour 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.inorgchem.9b03774 Ram Krishna Hona 1 , Farshid Ramezanipour 1
Affiliation
|
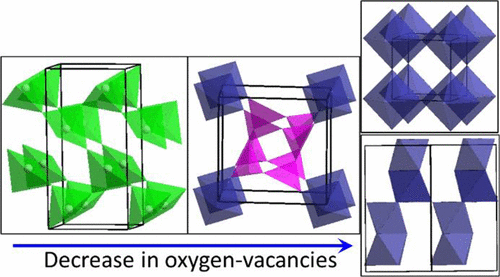
We report the facile synthesis methods of four materials, with the general formula SrMnO3−δ, which have previously been synthesized in multiple steps, involving switching between different oxidizing and reducing gases, quenching, the use of zirconium metal as a reductant, etc. However, we have shown that it is possible to synthesize all of these materials by facile processes without unnecessary complications. In fact, we have found methods of synthesizing the oxygen-deficient phases in only one step. Given the diverse range of structures that are formed for SrMnO3−δ, we have investigated the correlations between the structural order and electrocatalytic activity for the oxygen evolution reaction (OER) of water splitting. We have uncovered a systematic trend in the OER activity, where the most oxygen-deficient compound, SrMnO2.5, which features square-pyramidal coordination geometry around manganese, shows the highest OER performance. The next OER activity belongs to SrMnO2.6, which contains both MnO5 trigonal bipyramids and MnO6 octahedra. SrMnO3(cubic), containing only corner-sharing MnO6 units, shows the third best OER performance. The least activity is observed in SrMnO3(hexagonal), featuring both face- and corner-sharing MnO6 octahedra. We have also studied the electrochemically active surface area, as well as the kinetics of OER for all four materials, and found that the trend in these properties is the same as the trend in the OER activity. These findings indicate that the electrocatalytic activity is correlated with the degree of oxygen deficiency, as well as the polyhedral connectivity.
中文翻译:

氧空位和结构顺序对氧释放活性的影响:以具有四种不同结构类型的SrMnO 3−δ为例
我们报告了四种材料的简便合成方法,它们的通式为SrMnO3 -δ,以前已经通过多个步骤合成,包括在不同的氧化性和还原性气体之间切换,淬灭,使用锆金属作为还原剂等。但是,我们已经表明,可以通过简便的方法来合成所有这些材料而没有不必要的复杂性。实际上,我们发现仅一步就可以合成缺氧相的方法。给定SrMnO 3−δ形成的结构的不同范围,我们已经研究了水分解的氧释放反应(OER)的结构顺序与电催化活性之间的相关性。我们发现了OER活性的系统趋势,其中最缺氧的化合物SrMnO 2.5具有锰附近的方形-金字塔形配位几何结构,显示出最高的OER性能。下一个OER活性属于SrMnO 2.6,它同时包含MnO 5三角双锥和MnO 6八面体。仅包含转角共享MnO 6单元的SrMnO 3(立方)显示出第三好的OER性能。在SrMnO 3中观察到的活性最小(六边形),具有面共享和角共享的MnO 6八面体。我们还研究了所有四种材料的电化学活性表面积以及OER动力学,发现这些性质的趋势与OER活性的趋势相同。这些发现表明,电催化活性与缺氧程度以及多面体连接性相关。
更新日期:2020-03-27
中文翻译:

氧空位和结构顺序对氧释放活性的影响:以具有四种不同结构类型的SrMnO 3−δ为例
我们报告了四种材料的简便合成方法,它们的通式为SrMnO3 -δ,以前已经通过多个步骤合成,包括在不同的氧化性和还原性气体之间切换,淬灭,使用锆金属作为还原剂等。但是,我们已经表明,可以通过简便的方法来合成所有这些材料而没有不必要的复杂性。实际上,我们发现仅一步就可以合成缺氧相的方法。给定SrMnO 3−δ形成的结构的不同范围,我们已经研究了水分解的氧释放反应(OER)的结构顺序与电催化活性之间的相关性。我们发现了OER活性的系统趋势,其中最缺氧的化合物SrMnO 2.5具有锰附近的方形-金字塔形配位几何结构,显示出最高的OER性能。下一个OER活性属于SrMnO 2.6,它同时包含MnO 5三角双锥和MnO 6八面体。仅包含转角共享MnO 6单元的SrMnO 3(立方)显示出第三好的OER性能。在SrMnO 3中观察到的活性最小(六边形),具有面共享和角共享的MnO 6八面体。我们还研究了所有四种材料的电化学活性表面积以及OER动力学,发现这些性质的趋势与OER活性的趋势相同。这些发现表明,电催化活性与缺氧程度以及多面体连接性相关。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号