当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Prostratin, a Bioactive Tigliane Diterpenoid: Access to Multi-Stereocenter Cyclohexanes from a Phenol
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.0c00022 Guanghu Tong 1 , Zhengwei Ding 1 , Zhi Liu 1 , You-Song Ding 1 , Liang Xu 2 , Hailong Zhang 3 , Pengfei Li 1, 4
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.joc.0c00022 Guanghu Tong 1 , Zhengwei Ding 1 , Zhi Liu 1 , You-Song Ding 1 , Liang Xu 2 , Hailong Zhang 3 , Pengfei Li 1, 4
Affiliation
|
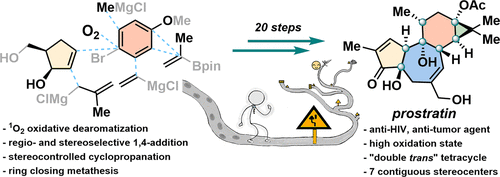
Tiglianes such as prostratin and related diterpenoids are biologically significant natural molecules and long-standing targets for organic synthesis community. Due to the complex polycyclic scaffolds, high oxygenation level, and dense functional groups and stereocenters, their de novo chemical syntheses still face formidable challenges despite extensive efforts in the past 40 years. This account details the development of a modular and concise synthesis of prostratin, a potent anti-HIV and anticancer agent. The key approach in this synthesis involved a sequence of oxidative dearomatization and sequential stereoselective installation of peripheral groups to rapidly build the contiguously substituted cyclohexane C-ring. Inspired by Wender’s work, an acid- and solvent-controlled stereodivergent formation of cyclopropane D-ring was developed. Mechanistic investigations by computational methods revealed that the competition between intra- and intermolecular hydrogen bonding led to different conformations, thus favoring different protonation processes. The designed and unexpected chemistry along this campaign reflected the uniqueness of the natural structures and should be amenable to future chemical syntheses of related complex polycyclic molecules.
中文翻译:

Prostratin的全合成,一种生物活性的Tigliane双萜类化合物:从苯酚中获得多立体中心的环己烷
Tiglianes(例如前驱素和相关的二萜类化合物)是生物学上重要的天然分子,也是有机合成社区的长期目标。由于复杂的多环支架,高氧化水平以及密集的官能团和立体中心,尽管在过去的40年中付出了巨大的努力,它们的从头化学合成仍然面临着严峻的挑战。该报告详细介绍了模块化和简明的prostratin(一种有效的抗HIV和抗癌药)合成方法的开发。该合成中的关键方法涉及一系列的氧化脱芳香化作用和外围基团的顺序立体选择性安装,以快速构建连续取代的环己烷C环。在温德(Wender)的工作启发下,开发了一种由酸和溶剂控制的立体发散型环丙烷D环。通过计算方法进行的机理研究表明,分子内和分子间氢键之间的竞争导致不同的构象,因此有利于不同的质子化过程。这项运动中设计和出乎意料的化学反应反映了天然结构的独特性,应该适合未来有关复杂多环分子的化学合成。
更新日期:2020-03-24
中文翻译:

Prostratin的全合成,一种生物活性的Tigliane双萜类化合物:从苯酚中获得多立体中心的环己烷
Tiglianes(例如前驱素和相关的二萜类化合物)是生物学上重要的天然分子,也是有机合成社区的长期目标。由于复杂的多环支架,高氧化水平以及密集的官能团和立体中心,尽管在过去的40年中付出了巨大的努力,它们的从头化学合成仍然面临着严峻的挑战。该报告详细介绍了模块化和简明的prostratin(一种有效的抗HIV和抗癌药)合成方法的开发。该合成中的关键方法涉及一系列的氧化脱芳香化作用和外围基团的顺序立体选择性安装,以快速构建连续取代的环己烷C环。在温德(Wender)的工作启发下,开发了一种由酸和溶剂控制的立体发散型环丙烷D环。通过计算方法进行的机理研究表明,分子内和分子间氢键之间的竞争导致不同的构象,因此有利于不同的质子化过程。这项运动中设计和出乎意料的化学反应反映了天然结构的独特性,应该适合未来有关复杂多环分子的化学合成。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号