Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The copper‐responsive ScsC protein of Salmonella promotes intramacrophage survival and interacts with the arginine sensor ArtI
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-09 , DOI: 10.1111/febs.15285 Buke Yucel 1 , Gary K. Robinson 1 , Mark Shepherd 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2020-03-09 , DOI: 10.1111/febs.15285 Buke Yucel 1 , Gary K. Robinson 1 , Mark Shepherd 1
Affiliation
|
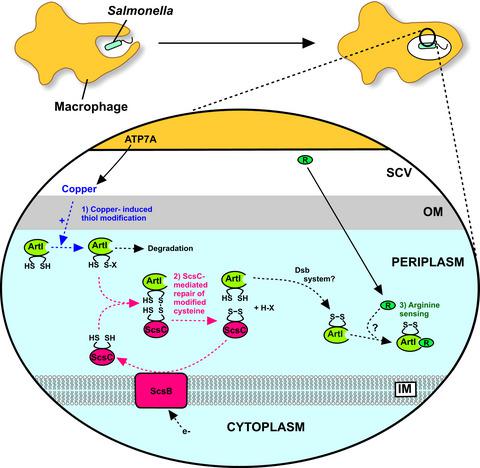
The scsABCD (suppressor of copper sensitivity) locus of Salmonella encodes four proteins that resemble the disulfide folding machinery of other bacteria. Previous work has shown that Salmonella encounters toxic levels of copper during infection and the Scs system provides protection against this copper‐mediated toxicity. The current work reports that expression of the soluble periplasmic protein StScsC is induced by copper and that intramacrophage survival in the presence of copper is diminished by the loss of StScsC. Using a combination of genetic and proteomic approaches, the abundance of various cysteine‐containing periplasmic proteins was found to be elevated by StScsC in the Salmonella periplasm, implicating StScsC in the disulfide folding of superoxide dismutases and proteins involved in amino acid sensing and import. Co‐purification and mass spectrometry approaches confirmed that the arginine‐sensing periplasmic protein ArtI associates with StScsC via a disulfide interaction, and purified ArtI was shown to alter the thiol redox state of purified StScsC. This work reports the first demonstration of a redox partner for the Scs system of Salmonella and provides insights into how this bacterial pathogen responds to copper stress during infection.
中文翻译:

沙门氏菌的铜响应性ScsC蛋白可促进巨噬细胞内存活并与精氨酸传感器ArtI相互作用
沙门氏菌的scsABCD(铜敏感性抑制剂)基因座编码四种蛋白质,类似于其他细菌的二硫键折叠机制。先前的工作表明,沙门氏菌在感染过程中会遇到铜的毒性水平,而Scs系统可提供针对这种铜介导的毒性的保护作用。目前的工作报道了可溶性的周质蛋白StScsC的表达是由铜诱导的,并且由于铜的存在而使StScsC的丧失降低了巨噬细胞在铜存在下的存活率。结合遗传和蛋白质组学方法,发现沙门氏菌中的StScsC可提高各种含半胱氨酸的周质蛋白的含量周质中,StScsC参与了涉及氨基酸传感和输入的超氧化物歧化酶和蛋白质的二硫键折叠。共纯化和质谱方法证实了精氨酸敏感的周质蛋白ArtI通过二硫键相互作用与StScsC缔合,纯化的ArtI被证明可以改变纯化的StScsC的硫醇氧化还原状态。这项工作报告了沙门氏菌Scs系统氧化还原伙伴的首次展示,并提供了对这种细菌病原体在感染过程中如何响应铜胁迫的见解。
更新日期:2020-03-09
中文翻译:

沙门氏菌的铜响应性ScsC蛋白可促进巨噬细胞内存活并与精氨酸传感器ArtI相互作用
沙门氏菌的scsABCD(铜敏感性抑制剂)基因座编码四种蛋白质,类似于其他细菌的二硫键折叠机制。先前的工作表明,沙门氏菌在感染过程中会遇到铜的毒性水平,而Scs系统可提供针对这种铜介导的毒性的保护作用。目前的工作报道了可溶性的周质蛋白StScsC的表达是由铜诱导的,并且由于铜的存在而使StScsC的丧失降低了巨噬细胞在铜存在下的存活率。结合遗传和蛋白质组学方法,发现沙门氏菌中的StScsC可提高各种含半胱氨酸的周质蛋白的含量周质中,StScsC参与了涉及氨基酸传感和输入的超氧化物歧化酶和蛋白质的二硫键折叠。共纯化和质谱方法证实了精氨酸敏感的周质蛋白ArtI通过二硫键相互作用与StScsC缔合,纯化的ArtI被证明可以改变纯化的StScsC的硫醇氧化还原状态。这项工作报告了沙门氏菌Scs系统氧化还原伙伴的首次展示,并提供了对这种细菌病原体在感染过程中如何响应铜胁迫的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号