当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cancer-associated fibroblasts that restrain cancer progression: Hypotheses and perspectives.
Cancer Science ( IF 4.5 ) Pub Date : 2020-03-10 , DOI: 10.1111/cas.14346 Yuki Miyai 1 , Nobutoshi Esaki 1 , Masahide Takahashi 1 , Atsushi Enomoto 1
Cancer Science ( IF 4.5 ) Pub Date : 2020-03-10 , DOI: 10.1111/cas.14346 Yuki Miyai 1 , Nobutoshi Esaki 1 , Masahide Takahashi 1 , Atsushi Enomoto 1
Affiliation
|
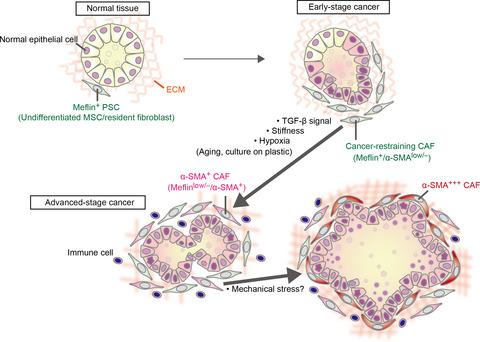
The roles of cancer-associated fibroblasts (CAF) in the progression of various types of cancers are well established. CAF promote cancer progression through pleiotropic mechanisms, including the secretion of soluble factors and extracellular matrix, physical interactions with cancer cells, and the regulation of angiogenesis, immunity and metabolism. Their contribution to therapeutic resistance is also well appreciated. Therefore, CAF have been considered as a therapeutic target in cancer. However, recent studies in autochthonous pancreatic cancer models suggest that specific subset(s) of CAF exhibit cancer-restraining roles, indicating that CAF are functionally and molecularly heterogeneous, which is supported by recent single-cell transcriptome analyses. While cancer-promoting CAF (pCAF) have been extensively studied, the nature and specific marker(s) of cancer-restraining CAF (rCAF) have remained uncharacterized. Interestingly, a recent study provided insight into the nature of rCAF and suggested that they may share molecular properties with pancreatic stellate cells (PSC) and mesenchymal stem/stromal cells (MSC). Complicating this finding is that PSC and MSC have been shown to promote the formation of a tumor-permissive and tumor-promoting environment in xenograft tumor models. However, these cells undergo significant transcriptional and epigenetic changes during ex vivo culture, which confounds the interpretation of experimental results based on the use of cultured cells. In this short review, we describe recent studies and hypotheses on the identity of rCAF and discuss their analogy to fibroblasts that suppress fibrosis in fibrotic diseases. Finally, we discuss how these findings can be exploited to develop novel anticancer therapies in the future.
中文翻译:

抑制癌症进展的与癌症相关的成纤维细胞:假设和观点。
癌症相关的成纤维细胞(CAF)在各种类型的癌症进展中的作用已得到充分确立。CAF通过多效性机制(包括可溶性因子和细胞外基质的分泌,与癌细胞的物理相互作用以及血管生成,免疫力和代谢的调节)促进癌症进展。它们对治疗抗性的贡献也被很好地理解。因此,CAF被认为是癌症的治疗靶标。但是,最近在本地胰腺癌模型中的研究表明,CAF的特定子集显示出抑制癌症的作用,表明CAF在功能和分子上是异质的,这受到最近的单细胞转录组分析的支持。尽管对促进癌症的CAF(pCAF)进行了广泛的研究,抑制癌症的CAF(rCAF)的性质和特异性标记仍未鉴定。有趣的是,最近的一项研究提供了对rCAF性质的洞察力,并暗示它们可能与胰腺星状细胞(PSC)和间充质干/基质细胞(MSC)共享分子特性。使这一发现复杂化的是,在异种移植肿瘤模型中,PSC和MSC已显示出促进肿瘤允许和促进肿瘤形成的环境。但是,这些细胞在离体培养过程中会发生明显的转录和表观遗传变化,这混淆了基于使用培养细胞对实验结果的解释。在这篇简短的综述中,我们描述了有关rCAF身份的最新研究和假设,并讨论了它们与抑制纤维化疾病中纤维化的成纤维细胞的相似性。最后,
更新日期:2020-04-22
中文翻译:

抑制癌症进展的与癌症相关的成纤维细胞:假设和观点。
癌症相关的成纤维细胞(CAF)在各种类型的癌症进展中的作用已得到充分确立。CAF通过多效性机制(包括可溶性因子和细胞外基质的分泌,与癌细胞的物理相互作用以及血管生成,免疫力和代谢的调节)促进癌症进展。它们对治疗抗性的贡献也被很好地理解。因此,CAF被认为是癌症的治疗靶标。但是,最近在本地胰腺癌模型中的研究表明,CAF的特定子集显示出抑制癌症的作用,表明CAF在功能和分子上是异质的,这受到最近的单细胞转录组分析的支持。尽管对促进癌症的CAF(pCAF)进行了广泛的研究,抑制癌症的CAF(rCAF)的性质和特异性标记仍未鉴定。有趣的是,最近的一项研究提供了对rCAF性质的洞察力,并暗示它们可能与胰腺星状细胞(PSC)和间充质干/基质细胞(MSC)共享分子特性。使这一发现复杂化的是,在异种移植肿瘤模型中,PSC和MSC已显示出促进肿瘤允许和促进肿瘤形成的环境。但是,这些细胞在离体培养过程中会发生明显的转录和表观遗传变化,这混淆了基于使用培养细胞对实验结果的解释。在这篇简短的综述中,我们描述了有关rCAF身份的最新研究和假设,并讨论了它们与抑制纤维化疾病中纤维化的成纤维细胞的相似性。最后,






























 京公网安备 11010802027423号
京公网安备 11010802027423号