当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Hydration and Hydroxyl Configurations of Gibbsite and Boehmite Nanoplates
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-19 , DOI: 10.1021/acs.jpcc.0c00659 Michel Sassi 1 , Zheming Wang 1 , Eric D. Walter 2 , Xin Zhang 1 , Hailin Zhang 1 , Xiaohong S. Li 3 , Aashish Tuladhar 1 , Mark Bowden 2 , Hong-Fei Wang 4 , Sue B. Clark 3, 5 , Kevin M. Rosso 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-02-19 , DOI: 10.1021/acs.jpcc.0c00659 Michel Sassi 1 , Zheming Wang 1 , Eric D. Walter 2 , Xin Zhang 1 , Hailin Zhang 1 , Xiaohong S. Li 3 , Aashish Tuladhar 1 , Mark Bowden 2 , Hong-Fei Wang 4 , Sue B. Clark 3, 5 , Kevin M. Rosso 1
Affiliation
|
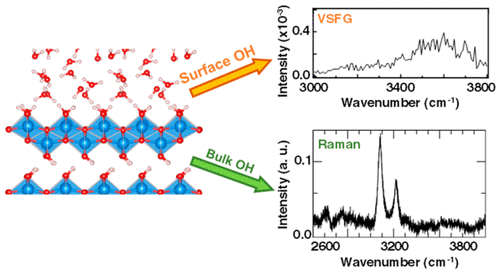
The discontinuation of the bulk structure at the interface between metal oxide particles and water leads to altered bonding characteristics and unique facet-dependent molecular environments. Surface hydration and hydroxylation add further complexity to the interface, details that for metal (oxy)hydroxides are especially difficult to isolate from the background signal of bulk structural hydroxyls. Here, we probe for the first time the surface hydroxyl structures and the effect of hydration on basal surfaces of gibbsite (α-Al(OH)3) and boehmite (γ-AlOOH) nanoplates under ambient conditions, using the interface-sensitive technique vibrational sum frequency generation (VSFG) spectroscopy. VSFG spectra of the hydroxyl stretching modes at the interfaces with adsorbed water layers compared directly to Raman and infrared spectra of the bulk modes show that while gibbsite surface frequencies were sharp and nearly identical to those in the bulk, boehmite surface hydroxyls displayed a very different broad spectrum of states. Ab initio molecular dynamics simulations of both basal surfaces with and without hydration waters reveal that gibbsite surface hydroxyls interact only weakly with overlying hydration waters remaining essentially unperturbed, whereas those on boehmite can interact more strongly facilitated by higher configurational degrees of freedom at the interface and there is more extensive H-bonding on boehmite surface under ambient conditions. The findings clearly unveil substantial differences in the hydrated interfacial dynamics of these two otherwise similar materials, with implications for their interfacial chemistry, wettability, and rheology.
中文翻译:

菱镁矿和勃姆石纳米板的表面水合和羟基构型
在金属氧化物颗粒和水之间的界面处的本体结构的中断导致改变的键合特性和独特的取决于面的分子环境。表面水合和羟基化进一步增加了界面的复杂性,详细说明了金属(羟基)氢氧化物尤其难以从本体结构羟基的背景信号中分离出来。在这里,我们首次探究了三水铝石(α-Al(OH)3的表面羟基结构和水合作用对基表面的影响)和勃姆石(γ-AlOOH)纳米板在环境条件下,使用界面敏感技术振动和频率生成(VSFG)光谱。直接与拉曼光谱相比较,在具有吸附水层的界面上的羟基拉伸模式的VSFG光谱和本体模式的红外光谱显示,三水铝石的表面频率尖锐且几乎与本体中的频率相同,而勃姆石的表面羟基具有宽广的差异状态范围。在有和没有水合水的情况下,两个基面的从头算分子动力学模拟表明,三水铝石表面的羟基与上覆的水合水几乎没有干扰,相互作用很小,而勃姆石上的那些可以通过界面处更高的构型自由度更强地相互作用,并且在环境条件下勃姆石表面上存在更广泛的氢键。这些发现清楚地揭示了这两种原本相似的材料在水合界面动力学方面的实质性差异,对其界面化学,润湿性和流变学具有影响。
更新日期:2020-02-19
中文翻译:

菱镁矿和勃姆石纳米板的表面水合和羟基构型
在金属氧化物颗粒和水之间的界面处的本体结构的中断导致改变的键合特性和独特的取决于面的分子环境。表面水合和羟基化进一步增加了界面的复杂性,详细说明了金属(羟基)氢氧化物尤其难以从本体结构羟基的背景信号中分离出来。在这里,我们首次探究了三水铝石(α-Al(OH)3的表面羟基结构和水合作用对基表面的影响)和勃姆石(γ-AlOOH)纳米板在环境条件下,使用界面敏感技术振动和频率生成(VSFG)光谱。直接与拉曼光谱相比较,在具有吸附水层的界面上的羟基拉伸模式的VSFG光谱和本体模式的红外光谱显示,三水铝石的表面频率尖锐且几乎与本体中的频率相同,而勃姆石的表面羟基具有宽广的差异状态范围。在有和没有水合水的情况下,两个基面的从头算分子动力学模拟表明,三水铝石表面的羟基与上覆的水合水几乎没有干扰,相互作用很小,而勃姆石上的那些可以通过界面处更高的构型自由度更强地相互作用,并且在环境条件下勃姆石表面上存在更广泛的氢键。这些发现清楚地揭示了这两种原本相似的材料在水合界面动力学方面的实质性差异,对其界面化学,润湿性和流变学具有影响。






























 京公网安备 11010802027423号
京公网安备 11010802027423号