当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Corannulene-Based Electron Acceptors: Combining Modular and Practical Synthesis with Electron Affinity and Solubility.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-23 , DOI: 10.1002/chem.201905521 Viktor Barát 1 , Maja Budanovic 1 , Si Man Tam 1 , June Huh 2 , Richard D Webster 1 , Mihaiela C Stuparu 1, 3
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-23 , DOI: 10.1002/chem.201905521 Viktor Barát 1 , Maja Budanovic 1 , Si Man Tam 1 , June Huh 2 , Richard D Webster 1 , Mihaiela C Stuparu 1, 3
Affiliation
|
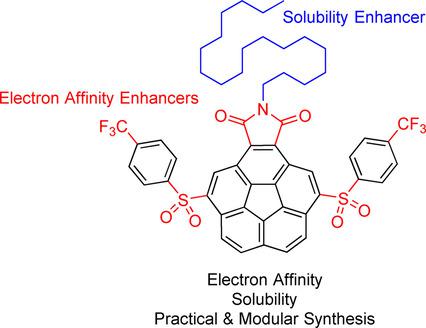
It is shown in this work that high electron affinity can be combined with high solubility and practical accessibility in corannulene-based electron acceptors. The electron affinity originates from the presence of three different types of electron-withdrawing groups (imide, sulfone, and trifluoromethyl) on the aromatic scaffold. The imide substituent further hosts a long alkyl chain (C18 H37 ) to boast solubility in a wide range of organic solvents. The synthesis is modular and consists of three simple steps from a commonly available corannulene derivative with an overall isolated yield of 22-27 %.
中文翻译:

基于Corannulene的电子受体:结合模块化和实用的合成与电子亲和力和溶解度。
在这项工作中表明,可以将高电子亲和力与高溶解度和实用性结合在基于环戊烯的电子受体中。电子亲和力源于芳族支架上三种不同类型的吸电子基团(酰亚胺,砜和三氟甲基)的存在。酰亚胺取代基还具有较长的烷基链(C18 H37),以在各种有机溶剂中具有较高的溶解度。合成是模块化的,由三个简单步骤组成,这些步骤由通常可得的香兰烯衍生物制成,总分离产率为22-27%。
更新日期:2020-02-19
中文翻译:

基于Corannulene的电子受体:结合模块化和实用的合成与电子亲和力和溶解度。
在这项工作中表明,可以将高电子亲和力与高溶解度和实用性结合在基于环戊烯的电子受体中。电子亲和力源于芳族支架上三种不同类型的吸电子基团(酰亚胺,砜和三氟甲基)的存在。酰亚胺取代基还具有较长的烷基链(C18 H37),以在各种有机溶剂中具有较高的溶解度。合成是模块化的,由三个简单步骤组成,这些步骤由通常可得的香兰烯衍生物制成,总分离产率为22-27%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号