当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trimethylaluminum as the Metal Precursor for the Atomic Layer Etching of Al2O3 Using Sequential, Self-Limiting Thermal Reactions
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-04-26 00:00:00 , DOI: 10.1021/acs.chemmater.6b00111 Younghee Lee 1 , Jaime W. DuMont 1 , Steven M. George 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2016-04-26 00:00:00 , DOI: 10.1021/acs.chemmater.6b00111 Younghee Lee 1 , Jaime W. DuMont 1 , Steven M. George 1
Affiliation
|
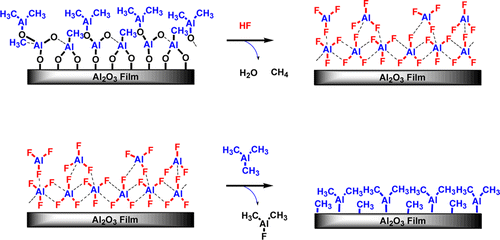
Trimethylaluminum (TMA, Al(CH3)3) was used as the metal precursor, together with HF, for the atomic layer etching (ALE) of Al2O3 using sequential, self-limiting thermal reactions. Al2O3 ALE using TMA demonstrates that other metal precursors, in addition to Sn(acac)2, can be employed for Al2O3 ALE. The use of TMA for Al2O3 ALE is especially interesting because TMA can also be used for Al2O3 atomic layer deposition (ALD). Quartz crystal microbalance (QCM) experiments monitored Al2O3 ALE at temperatures from 250 to 325 °C. The Al2O3 ALE was linear versus the number of HF and TMA reaction cycles. The QCM studies showed that the sequential HF and TMA reactions were self-limiting versus reactant exposure. The Al2O3 etching rates increased at higher temperatures. The QCM analysis measured mass change per cycle (MCPC) values that varied from −4.2 ng/(cm2 cycle) at 250 °C to −23.3 ng/(cm2 cycle) at 325 °C. These MCPCs correspond to Al2O3 etch rates from 0.14 Å/cycle at 250 °C to 0.75 Å/cycle at 325 °C. X-ray reflectivity and spectroscopic ellipsometry analyses confirmed the linear removal of Al2O3 and etching rates. Fourier transform infrared spectroscopy measurements monitored Al2O3 ALE by observing the loss of infrared absorbance from Al–O stretching vibrations. Surface intermediates were also identified after the HF and TMA exposures. Al2O3 ALE with TMA is believed to occur by the reaction Al2O3 + 4Al(CH3)3 + 6HF → 6AlF(CH3)2 + 3H2O. The proposed mechanism involves fluorination and ligand-exchange reactions. The HF exposure fluorinates the Al2O3 and forms an AlF3 surface layer and H2O as a volatile reaction product. During the ligand-exchange transmetalation reaction, TMA accepts F from the AlF3 surface layer and donates CH3 to produce volatile AlF(CH3)2 reaction products. The QCM measurements were consistent with an AlF3 surface layer thickness of 3.0 Å on Al2O3 after the HF exposures. The larger etch rates at higher temperatures were attributed to the removal of a larger fraction of the AlF3 surface layer by TMA exposures at higher temperatures.
中文翻译:

三甲基铝作为金属前体的连续自限热反应,用于Al 2 O 3原子层蚀刻
三甲基铝(TMA,Al(CH 3)3)与HF一起用作金属前体,用于通过顺序自限热反应对Al 2 O 3进行原子层蚀刻(ALE)。使用TMA的Al 2 O 3 ALE证明,除了Sn(acac)2以外,其他金属前体也可用于Al 2 O 3 ALE。将TMA用于Al 2 O 3 ALE特别有趣,因为TMA也可以用于Al 2 O 3原子层沉积(ALD)。石英晶体微天平(QCM)实验监测Al 2O 3 ALE在250至325°C的温度下。Al 2 O 3 ALE与HF和TMA反应循环数呈线性关系。QCM研究表明,顺序的HF和TMA反应与反应物暴露是自限性的。在较高温度下,Al 2 O 3蚀刻速率增加。QCM分析测量的每周期质量变化(MCPC)值从250°C时的-4.2 ng /(cm 2周期)到325°C时的-23.3 ng /(cm 2周期)变化。这些MCPC对应于Al 2 O 3蚀刻速率从250°C时的0.14Å/循环到325°C时的0.75Å/循环。X射线反射率和椭圆偏振光谱分析证实了Al 2 O 3的线性去除和蚀刻速率。傅立叶变换红外光谱测量通过观察Al–O拉伸振动引起的红外吸收损失来监测Al 2 O 3 ALE。在HF和TMA暴露后也鉴定出了表面中间体。据信具有TMA的Al 2 O 3 ALE通过反应Al 2 O 3 + 4Al(CH 3)3 + 6HF→6AlF(CH 3)2 + 3H 2发生O.拟议的机制涉及氟化和配体交换反应。所述HF曝光fluorinates所述Al 2 ö 3和形成的AlF 3的表面层和H 2 O作为挥发性反应产物。在配体交换金属过渡反应中,TMA从AlF 3表面层接受F并捐赠CH 3以产生挥发性AlF(CH 3)2反应产物。QCM测量与Al 2 O 3上的AlF 3表面层厚度为3.0Å一致HF暴露后。在较高温度下较大的蚀刻速率归因于在较高温度下通过TMA暴露去除了较大部分的AlF 3表面层。
更新日期:2016-04-26
中文翻译:

三甲基铝作为金属前体的连续自限热反应,用于Al 2 O 3原子层蚀刻
三甲基铝(TMA,Al(CH 3)3)与HF一起用作金属前体,用于通过顺序自限热反应对Al 2 O 3进行原子层蚀刻(ALE)。使用TMA的Al 2 O 3 ALE证明,除了Sn(acac)2以外,其他金属前体也可用于Al 2 O 3 ALE。将TMA用于Al 2 O 3 ALE特别有趣,因为TMA也可以用于Al 2 O 3原子层沉积(ALD)。石英晶体微天平(QCM)实验监测Al 2O 3 ALE在250至325°C的温度下。Al 2 O 3 ALE与HF和TMA反应循环数呈线性关系。QCM研究表明,顺序的HF和TMA反应与反应物暴露是自限性的。在较高温度下,Al 2 O 3蚀刻速率增加。QCM分析测量的每周期质量变化(MCPC)值从250°C时的-4.2 ng /(cm 2周期)到325°C时的-23.3 ng /(cm 2周期)变化。这些MCPC对应于Al 2 O 3蚀刻速率从250°C时的0.14Å/循环到325°C时的0.75Å/循环。X射线反射率和椭圆偏振光谱分析证实了Al 2 O 3的线性去除和蚀刻速率。傅立叶变换红外光谱测量通过观察Al–O拉伸振动引起的红外吸收损失来监测Al 2 O 3 ALE。在HF和TMA暴露后也鉴定出了表面中间体。据信具有TMA的Al 2 O 3 ALE通过反应Al 2 O 3 + 4Al(CH 3)3 + 6HF→6AlF(CH 3)2 + 3H 2发生O.拟议的机制涉及氟化和配体交换反应。所述HF曝光fluorinates所述Al 2 ö 3和形成的AlF 3的表面层和H 2 O作为挥发性反应产物。在配体交换金属过渡反应中,TMA从AlF 3表面层接受F并捐赠CH 3以产生挥发性AlF(CH 3)2反应产物。QCM测量与Al 2 O 3上的AlF 3表面层厚度为3.0Å一致HF暴露后。在较高温度下较大的蚀刻速率归因于在较高温度下通过TMA暴露去除了较大部分的AlF 3表面层。











































 京公网安备 11010802027423号
京公网安备 11010802027423号