Recently, the light-driven dual carbon research team at our institute, led by Dr. Li Yaguang, discovered that nickel single atomization can enhance the NH3 decomposition hydrogen production activity of nickel catalysts. They successfully coupled it with a photothermal system for efficient solar thermal ammonia gas decomposition hydrogen production. The related work, "Low Temperature Thermal and Solar Heating Carbon-Free Hydrogen Production from Ammonia Using Nickel Single Atom Catalysts," was published in Advanced Energy Materials (IF=29.698, 2022, 2202459) with Hebei University as the first institution. Dr. Li Yaguang is the first and corresponding author of the paper, and Dr. Zhang Zhibo and Professor Ye Jinhua are co-corresponding authors.
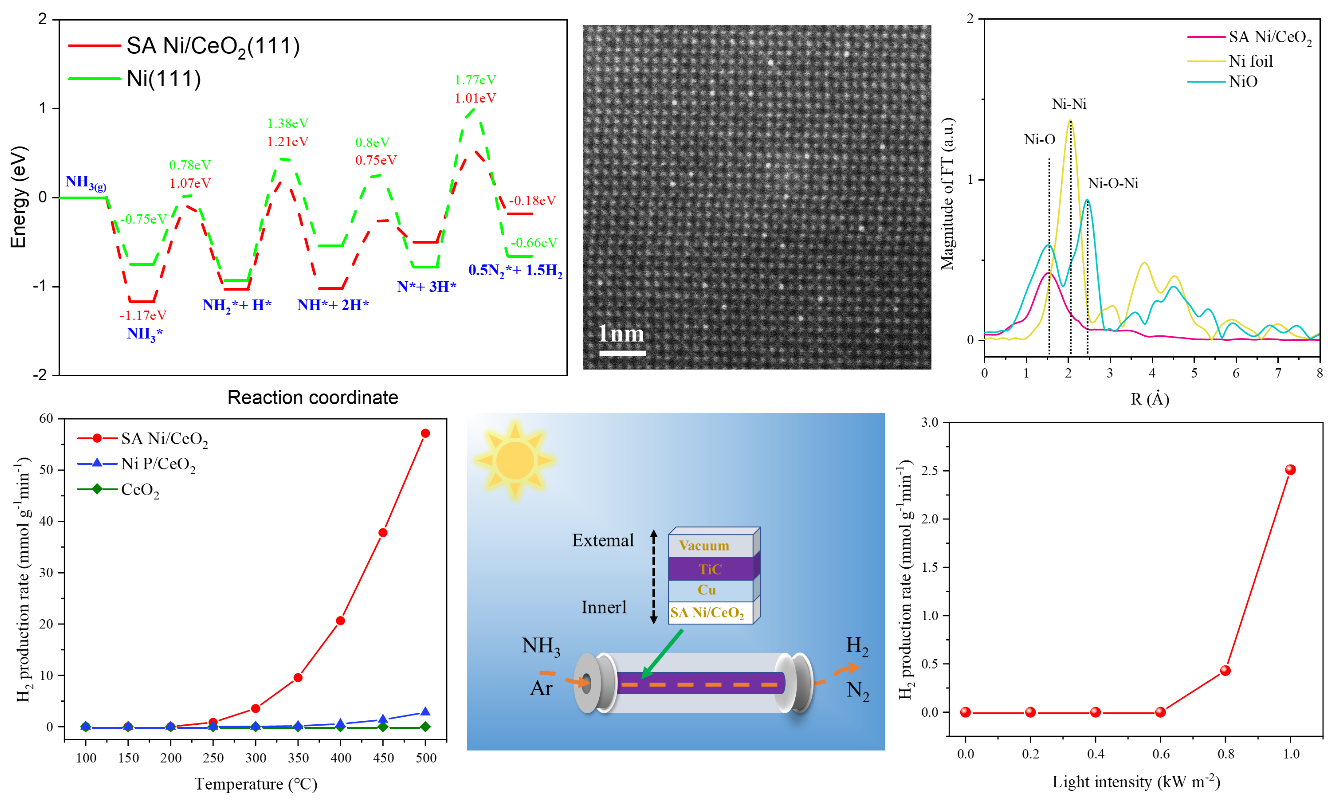
Catalytic ammonia decomposition hydrogen production is one of the fundamental technologies for constructing a zero-carbon hydrogen energy system, with non-precious metal materials such as nickel being the main catalysts for ammonia decomposition hydrogen production. Although various non-precious metal catalysts have been designed, their NH3 decomposition hydrogen production reaction still requires high temperatures (600-850°C), hindering the widespread application of ammonia decomposition hydrogen production. In this work, Li Yaguang and others, in collaboration with Professor Ye Jinhua and Dr. Zhang Zhibo, predicted through theoretical calculations that nickel single atoms can change the bonding mode of nickel catalysts with NH3, thereby enhancing the NH3 decomposition activity of nickel catalysts. Based on the theoretical results, the research team synthesized Ni single-atom/CeO2 two-dimensional material (SA Ni/CeO2) using the sol-gel method, demonstrating excellent NH3 catalytic decomposition performance at a low temperature of 300°C. Its H2 production rate was 3.544 mmol g-1 min-1, better than all non-precious metal catalysts and most precious metal catalysts. When combined with the custom photothermal device developed by the research group, under standard sunlight, the NH3 catalytic decomposition hydrogen production rate of SA Ni/CeO2 reached 1.58 mmol g-1 min-1, which is more than 100 times the reported natural sunlight-driven NH3 catalytic decomposition hydrogen production record rate, showing potential for practical application in zero-carbon hydrogen energy systems.
The above work was supported by the Hebei Provincial Natural Science Foundation, Hebei Provincial Department of Science and Technology, Hebei Provincial Department of Education, Hebei University Institute of Life Sciences and Green Development, Hebei University Multidisciplinary Natural Science Research Project, Hokkaido University's Photons Project, Guangdong Academy of Sciences, the State Key Laboratory of Catalysis, and the strong support of the Hebei University School of Physics Public Testing Center.
Paper link: https://doi.org/10.1002/aenm.202202459