当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural basis for binding of human IgG1 to its high-affinity human receptor FcγRI.
Nature Communications ( IF 14.7 ) Pub Date : 2015-Apr-30 , DOI: 10.1038/ncomms7866 Masato Kiyoshi , Jose M.M. Caaveiro , Takeaki Kawai , Shinya Tashiro , Teruhiko Ide , Yoshiharu Asaoka , Kouta Hatayama , Kouhei Tsumoto
Nature Communications ( IF 14.7 ) Pub Date : 2015-Apr-30 , DOI: 10.1038/ncomms7866 Masato Kiyoshi , Jose M.M. Caaveiro , Takeaki Kawai , Shinya Tashiro , Teruhiko Ide , Yoshiharu Asaoka , Kouta Hatayama , Kouhei Tsumoto
|
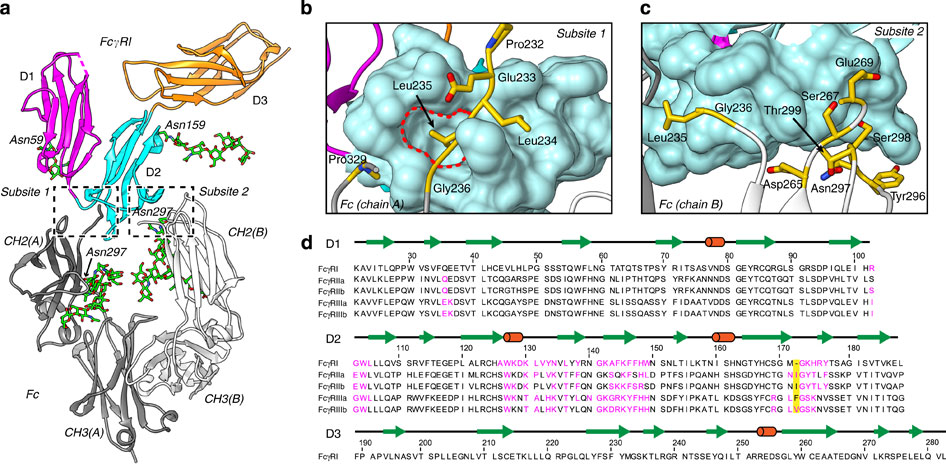
Cell-surface Fcγ receptors mediate innate and adaptive immune responses. Human Fcγ receptor I (hFcγRI) binds IgGs with high affinity and is the only Fcγ receptor that can effectively capture monomeric IgGs. However, the molecular basis of hFcγRI's interaction with Fc has not been determined, limiting our understanding of this major immune receptor. Here we report the crystal structure of a complex between hFcγRI and human Fc, at 1.80 Å resolution, revealing an unique hydrophobic pocket at the surface of hFcγRI perfectly suited for residue Leu235 of Fc, which explains the high affinity of this complex. Structural, kinetic and thermodynamic data demonstrate that the binding mechanism is governed by a combination of non-covalent interactions, bridging water molecules and the dynamic features of Fc. In addition, the hinge region of hFcγRI-bound Fc adopts a straight conformation, potentially orienting the Fab moiety. These findings will stimulate the development of novel therapeutic strategies involving hFcγRI.
中文翻译:

人IgG1与其高亲和力人类受体FcγRI结合的结构基础。
细胞表面的Fcγ受体介导先天性和适应性免疫反应。人Fcγ受体I(hFcγRI)以高亲和力结合IgG,并且是唯一可以有效捕获单体IgG的Fcγ受体。然而,尚未确定hFcγRI与Fc相互作用的分子基础,这限制了我们对这一主要免疫受体的理解。在这里,我们报告了hFcγRI与人Fc之间的复合物的晶体结构,分辨率为1.80Å,揭示了hFcγRI表面的独特疏水口袋,非常适合Fc的Leu235残基,这说明了该复合物的高亲和力。结构,动力学和热力学数据表明,结合机理受非共价相互作用,桥联水分子和Fc动力学特征的组合支配。此外,hFcγRI结合的Fc的铰链区采用直构象,潜在地定向Fab部分。这些发现将刺激涉及hFcγRI的新型治疗策略的发展。
更新日期:2015-05-04
中文翻译:

人IgG1与其高亲和力人类受体FcγRI结合的结构基础。
细胞表面的Fcγ受体介导先天性和适应性免疫反应。人Fcγ受体I(hFcγRI)以高亲和力结合IgG,并且是唯一可以有效捕获单体IgG的Fcγ受体。然而,尚未确定hFcγRI与Fc相互作用的分子基础,这限制了我们对这一主要免疫受体的理解。在这里,我们报告了hFcγRI与人Fc之间的复合物的晶体结构,分辨率为1.80Å,揭示了hFcγRI表面的独特疏水口袋,非常适合Fc的Leu235残基,这说明了该复合物的高亲和力。结构,动力学和热力学数据表明,结合机理受非共价相互作用,桥联水分子和Fc动力学特征的组合支配。此外,hFcγRI结合的Fc的铰链区采用直构象,潜在地定向Fab部分。这些发现将刺激涉及hFcγRI的新型治疗策略的发展。






























 京公网安备 11010802027423号
京公网安备 11010802027423号