当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ammonia electrooxidation on dendritic Pt nanostructures in alkaline solutions investigated by in-situ FTIR spectroscopy and online electrochemical mass spectroscopy
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.jelechem.2017.12.062 Jin-Yu Ye , Jian-Long Lin , Zhi-You Zhou , Yu-Hao Hong , Tian Sheng , Muhammad Rauf , Shi-Gang Sun
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-06-01 , DOI: 10.1016/j.jelechem.2017.12.062 Jin-Yu Ye , Jian-Long Lin , Zhi-You Zhou , Yu-Hao Hong , Tian Sheng , Muhammad Rauf , Shi-Gang Sun
|
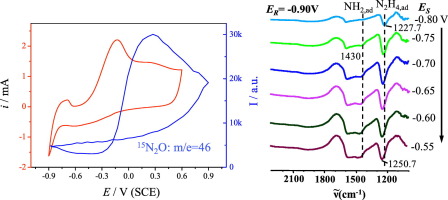
Abstract Dendritic Pt nanostructures were prepared through electrodeposition on a substrate Pt electrode by applying square-wave potential method. It has been found that the as-prepared dendritic Pt nanostructure exhibited enhanced IR absorption with an enhancement factor up to 10 folds for adsorbed CO species. Such an enhanced in-situ FTIR spectroscopy (FTIRS) has been applied in studies of reaction mechanism of ammonia electrooxidation. From in-situ FTIR spectra recorded during ammonia electrooxidation, two characteristic IR bands at 1430 cm− 1 and between 1227 and 1250 cm− 1 were observed at low potential region (E 0.10 V vs. SCE), two IR bands at 2231 cm− 1 and 1236 cm− 1 were observed and ascribed respectively to N2O and NO2− species, which are the ultimate oxidation products detected under present investigation conditions. In addition to in-situ FTIR spectroscopy, online electrochemical mass spectroscopy (OEMS) was used to detect volatile products. The clear OEMS signals of m/e = 30 and m/e = 46 measured at potentials above − 0.5 V and − 0.30 V, respectively, indicate the production of N2 (IR inactive) and confirm the generation of N2O. Based on results of cyclic voltammetry, in-situ FTIRS and OEMS, the reaction mechanism is therefore elucidated with molecular details of intermediates and products involved in ammonia electrooxidation in alkaline solutions.
中文翻译:

通过原位 FTIR 光谱和在线电化学质谱研究碱性溶液中树枝状 Pt 纳米结构的氨电氧化
摘要 采用方波电位法在基底Pt电极上电沉积制备树枝状Pt纳米结构。已经发现,所制备的树枝状 Pt 纳米结构表现出增强的 IR 吸收,对于吸附的 CO 物种,增强因子高达 10 倍。这种增强的原位 FTIR 光谱 (FTIRS) 已应用于氨电氧化反应机理的研究。从氨电氧化过程中记录的原位 FTIR 光谱中,在低电位区域(E 0.10 V vs. SCE)观察到 1430 cm− 1 和 1227 和 1250 cm− 1 之间的两个特征红外波段,2231 cm− 处的两个红外波段1 和 1236 cm- 1 被观察到并分别归因于 N2O 和 NO2- 物种,它们是在当前调查条件下检测到的最终氧化产物。除了原位 FTIR 光谱之外,在线电化学质谱 (OEMS) 还用于检测挥发性产品。m/e = 30 和 m/e = 46 的清晰 OEMS 信号分别在高于 − 0.5 V 和 − 0.30 V 的电位下测量,表明产生了 N2(IR 不活动)并确认了 N2O 的产生。因此,基于循环伏安法、原位 FTIRS 和 OEMS 的结果,通过碱性溶液中氨电氧化所涉及的中间体和产物的分子细节阐明了反应机理。
更新日期:2018-06-01
中文翻译:

通过原位 FTIR 光谱和在线电化学质谱研究碱性溶液中树枝状 Pt 纳米结构的氨电氧化
摘要 采用方波电位法在基底Pt电极上电沉积制备树枝状Pt纳米结构。已经发现,所制备的树枝状 Pt 纳米结构表现出增强的 IR 吸收,对于吸附的 CO 物种,增强因子高达 10 倍。这种增强的原位 FTIR 光谱 (FTIRS) 已应用于氨电氧化反应机理的研究。从氨电氧化过程中记录的原位 FTIR 光谱中,在低电位区域(E 0.10 V vs. SCE)观察到 1430 cm− 1 和 1227 和 1250 cm− 1 之间的两个特征红外波段,2231 cm− 处的两个红外波段1 和 1236 cm- 1 被观察到并分别归因于 N2O 和 NO2- 物种,它们是在当前调查条件下检测到的最终氧化产物。除了原位 FTIR 光谱之外,在线电化学质谱 (OEMS) 还用于检测挥发性产品。m/e = 30 和 m/e = 46 的清晰 OEMS 信号分别在高于 − 0.5 V 和 − 0.30 V 的电位下测量,表明产生了 N2(IR 不活动)并确认了 N2O 的产生。因此,基于循环伏安法、原位 FTIRS 和 OEMS 的结果,通过碱性溶液中氨电氧化所涉及的中间体和产物的分子细节阐明了反应机理。





























 京公网安备 11010802027423号
京公网安备 11010802027423号