JAMA Neurology ( IF 20.4 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaneurol.2017.2286 Xia Wang,Thompson G. Robinson,Tsong-Hai Lee,Qiang Li,Hisatomi Arima,Philip M. Bath,Laurent Billot,Joseph Broderick,Andrew M. Demchuk,Geoffrey Donnan,Jong S. Kim,Pablo Lavados,Richard I. Lindley,Sheila O. Martins,Veronica V. Olavarria,Jeyaraj D. Pandian,Mark W. Parsons,Octavio M. Pontes-Neto,Stefano Ricci,Vijay K. Sharma,Nguyen H. Thang,Ji-Guang Wang,Mark Woodward,Craig S. Anderson,John Chalmers,null null
|
Importance A lower dose of intravenous alteplase appears to be a safer treatment option than the standard dose, reducing the risk of symptomatic intracerebral hemorrhage. There is uncertainty, however, over how this effect translates into an overall clinical benefit for patients with acute ischemic stroke (AIS).
Objective To assess whether older, Asian, or severely affected patients with AIS who are considered at high risk of thrombolysis may benefit more from low-dose rather than standard-dose alteplase treatment.
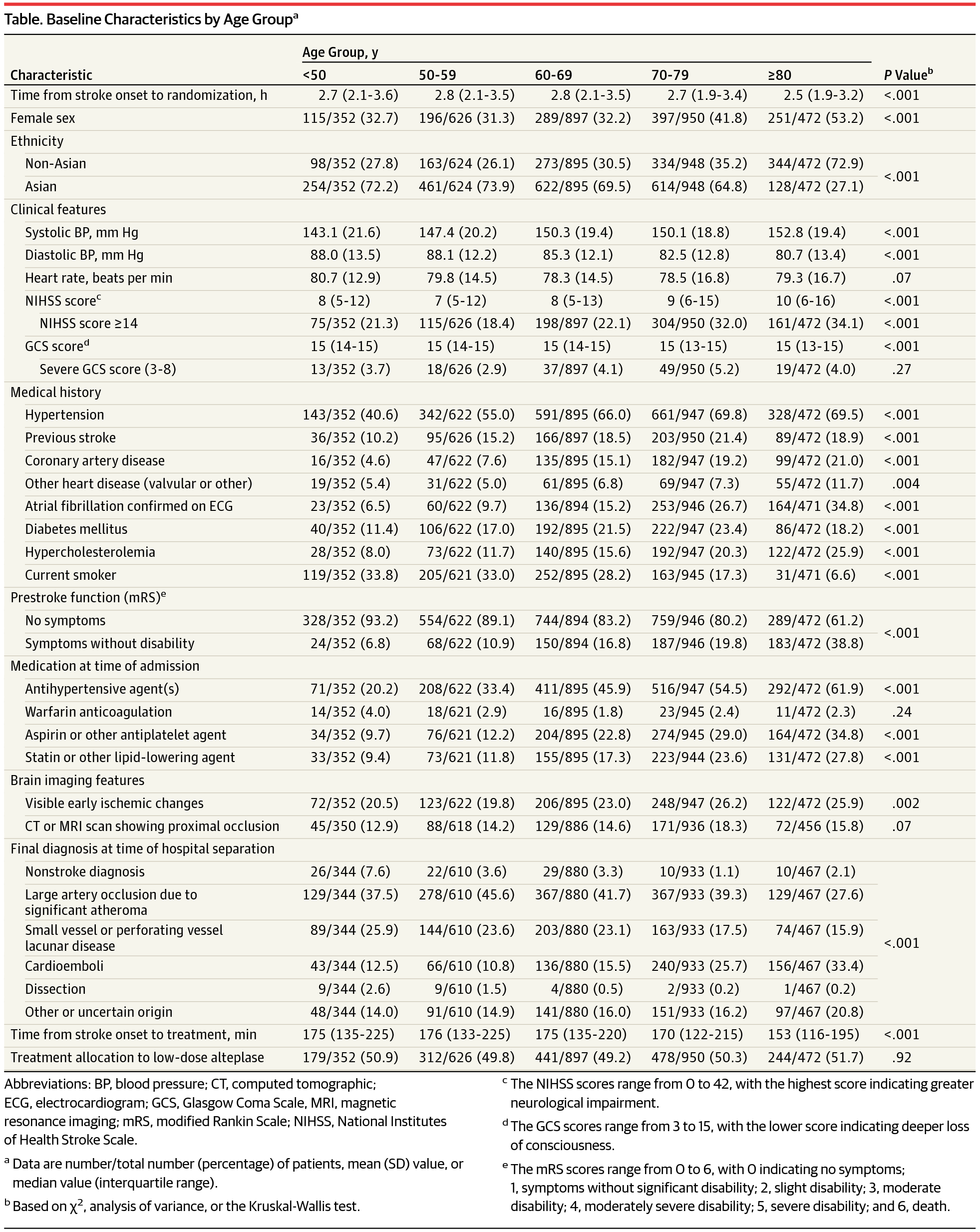
Design, Setting, and Participants This study is a prespecified secondary analysis of the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED), an international, randomized, open-label, blinded, end-point clinical trial of low-dose vs standard-dose intravenous alteplase for patients with AIS. From March 1, 2012, to August 31, 2015, a total of 3310 patients who had a clinical diagnosis of AIS as confirmed by brain imaging and who fulfilled the local criteria for thrombolysis treatment were included in the alteplase-dose arms. Patients were randomly assigned to receive low-dose (0.6 mg/kg; 15% as bolus and 85% as infusion over 1 hour) or standard-dose (0.9 mg/kg; 10% as bolus and 90% as infusion over 1 hour) alteplase. Of the 3310 randomized patients, 13 patients were excluded for missing consent, mistaken randomization, and duplicate randomization numbers. This secondary analysis was conducted between May 1, 2016, and April 28, 2017.
Main Outcomes and Measures The primary end point was a poor outcome defined by the combination of death and any disability as scored by the modified Rankin Scale (scores range from 2 to 6, with the highest score indicating death) at 90 days.
Results Of the 3297 patients included in the analysis, 1248 (37.9%) were women, and the mean (SD) age was 67 (13) years. No significant differences in the treatment effects were observed between low- and standard-dose alteplase for poor outcomes (death or disability) by age, ethnicity, or severity (all P > .37 for interaction). Similarly, the treatment effects of low- vs standard-dose alteplase on function outcome (ordinal shift of the modified Rankin Scale) in Asians (odds ratio, 1.05; 95% CI, 0.90-1.22) was consistent with non-Asians (odds ratio, 0.93; 95% CI, 0.76-1.14) (P = .32 for interaction). There were generally consistent reductions in rates of symptomatic intracerebral hemorrhage with low-dose alteplase, although this reduction was not statistically significant by age, ethnicity, or severity.
Conclusions and Relevance This analysis found that the effects of low-dose alteplase were not clearly superior to the effects of standard-dose alteplase on death or disability in key demographic subgroups of patients with AIS. Further investigation is required to identify patients with AIS who may benefit from low-dose alteplase.
Trial Registration clinicaltrials.gov Identifier: NCT01422616
中文翻译:

低剂量与标准剂量阿替普酶治疗急性缺血性卒中的随机对照临床试验的二级分析
重要性 较低剂量的静脉阿替普酶似乎是比标准剂量更安全的治疗选择,从而减少了症状性脑出血的风险。但是,对于急性缺血性中风(AIS)患者,这种作用如何转化为整体临床益处尚不确定。
目的 为了评估被认为具有高溶栓风险的老年,亚洲或重度感染的AIS患者是否可以从低剂量而非标准剂量的阿替普酶治疗中获益更多。
设计,设置和参与者 这项研究是对高血压和溶栓性卒中研究的增强控制(ENCHANTED)的一项预先指定的次要分析,这是一项针对低剂量与标准剂量静脉使用阿替普酶的国际性,随机,开放标签,盲法,终点临床试验AIS。从2012年3月1日至2015年8月31日,阿替普酶剂量治疗组共纳入3310例经脑影像学检查确诊为AIS并符合当地溶栓治疗标准的患者。随机分配患者接受小剂量(0.6 mg / kg;推注15%和1小时内输注的85%)或标准剂量(0.9 mg / kg;推注10%和1小时内输注的90%) )阿替普酶。在3310名随机分组的患者中,有13名患者因缺少同意,错误的随机分组和重复的随机数而被排除在外。
主要结果和衡量指标 主要终点是90天后死亡和不良反应的综合结果,该结果由死亡和任何残疾综合评分,评分方法采用改良的兰金量表(评分范围为2到6,最高分表示死亡)。
结果 分析的3297名患者中,女性为1248名(37.9%),平均(SD)年龄为67岁(13)。低剂量和标准剂量阿替普酶对不良预后(死亡或残疾)的年龄,种族或严重性( 相互作用的所有P > .37)均未观察到治疗效果的显着差异。同样,低剂量与标准剂量阿替普酶对亚洲人功能结局(改良的兰金评分的标准偏移)的治疗效果(比值比为1.05; 95%CI为0.90-1.22)与非亚洲人一致(比值比) ,0.93; 95%CI,0.76-1.14)(P = 0.32(用于互动)。小剂量阿替普酶治疗后症状性脑出血的发生率总体上持续降低,尽管按年龄,种族或严重程度而言,这种减少率在统计学上并不显着。
结论与相关性 该分析发现,低剂量阿替普酶对AIS患者关键人群亚组的死亡或致残性的影响并不明显优于标准剂量阿替普酶。需要进一步研究以鉴定可能受益于低剂量阿替普酶的AIS患者。
试验注册 临床试验.gov标识符:NCT01422616







































 京公网安备 11010802027423号
京公网安备 11010802027423号