当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Self-Assembly Pathways of β-Sheet-Rich Amyloid-β(1–40) Dimers: Markov State Model Analysis on Millisecond Hybrid-Resolution Simulations
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jctc.7b00803 Yang Cao 1 , Xuehan Jiang 1 , Wei Han 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1021/acs.jctc.7b00803 Yang Cao 1 , Xuehan Jiang 1 , Wei Han 1
Affiliation
|
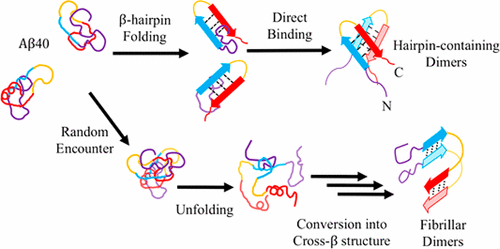
Early oligomerization during amyloid-β (Aβ) aggregation is essential for Aβ neurotoxicity. Understanding how unstructured Aβs assemble into oligomers, especially those rich in β-sheets, is essential but remains challenging as the assembly process is too transient for experimental characterization and too slow for molecular dynamics simulations. So far, atomic simulations are limited only to studies of either oligomer structures or assembly pathways for short Aβ segments. To overcome the computational challenge, we combine in this study a hybrid-resolution model and adaptive sampling techniques to perform over 2.7 ms of simulations of formation of full-length Aβ40 dimers that are the earliest toxic oligomeric species. The Markov state model is further employed to characterize the transition pathways and associated kinetics. Our results show that for two major forms of β-sheet-rich structures reported experimentally, the corresponding assembly mechanisms are markedly different. Hairpin-containing structures are formed by direct binding of soluble Aβ in β-hairpin-like conformations. Formation of parallel, in-register structures resembling fibrils occurs ∼100-fold more slowly and involves a rapid encounter of Aβ in arbitrary conformations followed by a slow structural conversion. The structural conversion proceeds via diverse pathways but always requires transient unfolding of encounter complexes. We find that the transition kinetics could be affected differently by intra-/intermolecular interactions involving individual residues in a conformation-dependent manner. In particular, the interactions involving Aβ’s N-terminal part promote the assembly into hairpin-containing structures but delay the formation of fibril-like structures, thus explaining puzzling observations reported previously regarding the roles of this region in the early assembly process.
中文翻译:

β-Sheet-Amyloid-β(1-40)二聚体的自组装途径:毫秒混合分辨模拟的马尔可夫状态模型分析
淀粉样蛋白-β(Aβ)聚集过程中的早期低聚化对于Aβ神经毒性至关重要。了解非结构化的Aβ如何组装成低聚物(尤其是富含β-折叠的低聚物)是至关重要的,但仍然具有挑战性,因为组装过程对于实验表征而言太短暂而对于分子动力学模拟而言太慢。到目前为止,原子模拟仅限于针对短Aβ片段的低聚物结构或组装途径的研究。为了克服计算难题,我们在这项研究中结合了混合分辨率模型和自适应采样技术,以进行2.7 ms以上的模拟,该模拟是最早的有毒低聚物种全长Aβ40二聚体的形成。马尔可夫状态模型被进一步用来表征过渡路径和相关的动力学。我们的结果表明,对于实验报告的两种主要形式的富含β-折叠的结构,相应的组装机理明显不同。含有发夹的结构是通过将可溶性Aβ直接结合成类似β-发夹的构象而形成的。类似于原纤维的平行,配准结构的形成发生的速度要慢100倍左右,并且涉及任意构象的Aβ快速相遇,然后进行缓慢的结构转化。结构转换是通过多种途径进行的,但始终需要遇到复合物的瞬时展开。我们发现过渡动力学可能受到构象依赖性方式涉及单个残基的分子内/分子间相互作用的影响。尤其是,
更新日期:2017-10-27
中文翻译:

β-Sheet-Amyloid-β(1-40)二聚体的自组装途径:毫秒混合分辨模拟的马尔可夫状态模型分析
淀粉样蛋白-β(Aβ)聚集过程中的早期低聚化对于Aβ神经毒性至关重要。了解非结构化的Aβ如何组装成低聚物(尤其是富含β-折叠的低聚物)是至关重要的,但仍然具有挑战性,因为组装过程对于实验表征而言太短暂而对于分子动力学模拟而言太慢。到目前为止,原子模拟仅限于针对短Aβ片段的低聚物结构或组装途径的研究。为了克服计算难题,我们在这项研究中结合了混合分辨率模型和自适应采样技术,以进行2.7 ms以上的模拟,该模拟是最早的有毒低聚物种全长Aβ40二聚体的形成。马尔可夫状态模型被进一步用来表征过渡路径和相关的动力学。我们的结果表明,对于实验报告的两种主要形式的富含β-折叠的结构,相应的组装机理明显不同。含有发夹的结构是通过将可溶性Aβ直接结合成类似β-发夹的构象而形成的。类似于原纤维的平行,配准结构的形成发生的速度要慢100倍左右,并且涉及任意构象的Aβ快速相遇,然后进行缓慢的结构转化。结构转换是通过多种途径进行的,但始终需要遇到复合物的瞬时展开。我们发现过渡动力学可能受到构象依赖性方式涉及单个残基的分子内/分子间相互作用的影响。尤其是,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号