当前位置:
X-MOL 学术
›
Food Res. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quercetin as a tyrosinase inhibitor: Inhibitory activity, conformational change and mechanism
Food Research International ( IF 7.0 ) Pub Date : 2017-07-04 , DOI: 10.1016/j.foodres.2017.07.010 Meihui Fan 1 , Guowen Zhang 1 , Xing Hu 1 , Ximing Xu 2 , Deming Gong 3
Food Research International ( IF 7.0 ) Pub Date : 2017-07-04 , DOI: 10.1016/j.foodres.2017.07.010 Meihui Fan 1 , Guowen Zhang 1 , Xing Hu 1 , Ximing Xu 2 , Deming Gong 3
Affiliation
|
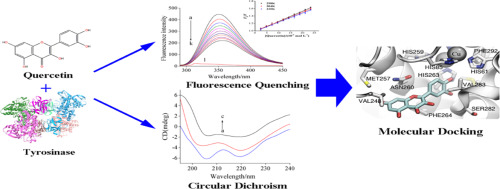
Quercetin, a flavonoid compound, was found to inhibit both monophenolase and diphenolase activities of tyrosinase, and its inhibition against diphenolase activity was in a reversible and competitive manner with an IC50 value of (3.08 ± 0.74) × 10− 5 mol L− 1. Quercetin bound to tyrosinase driven by hydrophobic interaction, thereby resulted in a conformational change of tyrosinase and its intrinsic fluorescence quenching. Tyrosinase had one binding site for quercetin with the binding constant in the order of magnitude of 104 L mol− 1. The molecular docking revealed that quercetin bound to the active site of tyrosinase and chelated a copper with the 3′, 4′-dihydroxy groups. It can be deduced that the chelation may prevent the entrance of substrate and then inhibit the catalytic activity of tyrosinase. These findings may be helpful to understand the inhibition mechanism of quercetin on tyrosinase and functional research of quercetin in the treatment of pigmentation disorders.
中文翻译:

槲皮素作为酪氨酸酶抑制剂:抑制活性、构象变化和机制
槲皮素是一种黄酮类化合物,对酪氨酸酶的单酚酶和双酚酶活性均有抑制作用,其对双酚酶活性的抑制是可逆的、竞争性的,IC 50值为 (3.08 ± 0.74) × 10 - 5 mol L - 1 . 槲皮素通过疏水相互作用与酪氨酸酶结合,从而导致酪氨酸酶的构象变化及其固有的荧光猝灭。酪氨酸酶有一个槲皮素结合位点,结合常数为 10 4 L mol - 1 . 分子对接显示槲皮素与酪氨酸酶的活性位点结合,并与 3'、4'-二羟基基团螯合铜。可以推断,螯合可以阻止底物的进入,进而抑制酪氨酸酶的催化活性。这些发现可能有助于了解槲皮素对酪氨酸酶的抑制机制以及槲皮素治疗色素沉着障碍的功能研究。
更新日期:2017-09-04
中文翻译:

槲皮素作为酪氨酸酶抑制剂:抑制活性、构象变化和机制
槲皮素是一种黄酮类化合物,对酪氨酸酶的单酚酶和双酚酶活性均有抑制作用,其对双酚酶活性的抑制是可逆的、竞争性的,IC 50值为 (3.08 ± 0.74) × 10 - 5 mol L - 1 . 槲皮素通过疏水相互作用与酪氨酸酶结合,从而导致酪氨酸酶的构象变化及其固有的荧光猝灭。酪氨酸酶有一个槲皮素结合位点,结合常数为 10 4 L mol - 1 . 分子对接显示槲皮素与酪氨酸酶的活性位点结合,并与 3'、4'-二羟基基团螯合铜。可以推断,螯合可以阻止底物的进入,进而抑制酪氨酸酶的催化活性。这些发现可能有助于了解槲皮素对酪氨酸酶的抑制机制以及槲皮素治疗色素沉着障碍的功能研究。

































 京公网安备 11010802027423号
京公网安备 11010802027423号