Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2017-07-28 , DOI: 10.1016/j.bmc.2017.07.044 Akihiro Tai , Atsuko Iomori , Hideyuki Ito
|
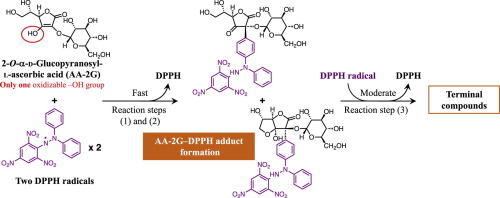
2-O-α-d-Glucopyranosyl-l-ascorbic acid (AA-2G) exhibits biological activities after enzymatic hydrolysis to ascorbic acid (AA) by α-glucosidase. We have found that AA-2G per se exerted radical-scavenging activity toward 1,1-diphenyl-2-picrylhydrazyl radical (DPPH radical). The radical-scavenging property of AA-2G was greatly different from that of AA; that is, the reaction rate with DPPH radical of AA-2G was far slower than that of AA, but the long-lasting radical-scavenging ability per one molecule of AA-2G was superior to that of AA. We purified key intermediates for the characteristic radical-scavenging reaction of AA-2G and carried out time-course studies of the radical-scavenging reactions of the intermediates, AA-2G and AA to determine both the reaction rate and stoichiometry of AA-2G with DPPH radical. One mole of AA-2G quenched 2.7 mol of DPPH radical over a period of 120 min, while one mole of AA quenched 1.9 mol of the radical. The high reaction stoichiometry of AA-2G against DPPH radical was associated with adduct formation of AA-2G with DPPH radical. The radical-scavenging reaction mechanism of AA-2G consists of the following three steps: (1) At an early stage of the reaction, AA-2G scavenged DPPH radical to generate AA-2G radical, (2) AA-2G radical immediately reacted with an additional DPPH radical to give two types of AA-2G–DPPH adducts and (3) AA-2G–DPPH adducts slowly quenched the other DPPH radical to generate several reaction products. Our results suggest the practical value of AA-2G, even before being converted into AA, as a beneficial antioxidant in food and cosmetic applications.
中文翻译:

2 - O -α-d-吡喃葡萄糖基-1-抗坏血酸的DPPH自由基清除机理的结构证据
2- ø -α- d -Glucopyranosyl-升抗坏血酸(AA-2G)具有由α葡糖苷酶水解成抗坏血酸(AA)后的生物活性。我们发现AA-2G本身对1,1-二苯基-2-吡啶并肼基(DPPH基)具有清除自由基的活性。AA-2G的自由基清除性质与AA的清除性质大不相同。也就是说,AA-2G与DPPH自由基的反应速率远慢于AA,但每1个分子AA-2G的持久自由基清除能力优于AA-2G。我们为AA-2G的特征自由基清除反应纯化了关键中间体,并对中间体AA-2G和AA的自由基清除反应进行了时程研究,以确定AA-2G的反应速率和化学计量DPPH自由基。一摩尔AA-2G在120分钟内淬灭了2.7摩尔的DPPH自由基,而一摩尔AA-2G淬灭了1.9摩尔的自由基。AA-2G对DPPH自由基的高反应化学计量与AA-2G与DPPH自由基的加合物形成有关。AA-2G的自由基清除反应机理包括以下三个步骤:(1)在反应的早期,AA-2G清除DPPH自由基以生成AA-2G自由基,(2)AA-2G自由基立即反应与一个额外的DPPH自由基产生两种类型的AA-2G-DPPH加合物,以及(3)AA-2G-DPPH加合物缓慢淬灭另一个DPPH自由基以生成几种反应产物。我们的结果表明,即使在转化为AA之前,AA-2G在食品和化妆品应用中作为有益的抗氧化剂也具有实用价值。AA-2G清除了DPPH自由基以生成AA-2G自由基,(2)AA-2G自由基立即与另一个DPPH自由基反应生成两种类型的AA-2G-DPPH加合物,(3)AA-2G-DPPH加合物缓慢淬灭另一个DPPH自由基产生几种反应产物。我们的结果表明,即使在转化为AA之前,AA-2G在食品和化妆品应用中作为有益的抗氧化剂也具有实用价值。AA-2G清除了DPPH自由基以生成AA-2G自由基,(2)AA-2G自由基立即与另外的DPPH自由基反应生成两种类型的AA-2G-DPPH加合物,(3)AA-2G-DPPH缓慢淬灭另一个DPPH自由基产生几种反应产物。我们的结果表明,即使在转化为AA之前,AA-2G在食品和化妆品应用中作为有益的抗氧化剂也具有实用价值。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号