当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Eriodictyol, Not Its Glucuronide Metabolites, Attenuates Acetaminophen-Induced Hepatotoxicity
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00345 Zhaoyu Wang 1 , Yao Lan 1 , MingHao Chen 1 , Cailing Wen 1 , Yanxian Hu 1 , Zhongqiu Liu 1, 2 , Ling Ye 1, 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-07-17 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00345 Zhaoyu Wang 1 , Yao Lan 1 , MingHao Chen 1 , Cailing Wen 1 , Yanxian Hu 1 , Zhongqiu Liu 1, 2 , Ling Ye 1, 3
Affiliation
|
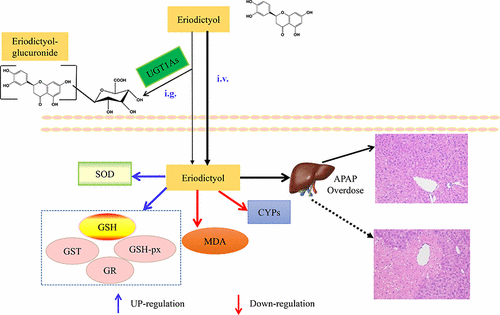
Acetaminophen (APAP) is one of the most commonly used oral analgesics and antipyretics, but hepatotoxicity including liver failure may occur after overdose. The therapeutic options for treating APAP hepatotoxicity are limited. Eriodictyol, a dietary flavonoid with anti-inflammatory and antioxidant properties, was used here to determine its protective effects against APAP-induced hepatotoxicity in mice. Various administration routes and pharmacokinetics–pharmacodynamics (PK–PD) analyses were used to determine these effects. Protective effects were observed in intravenously and intraperitoneally but not in intragastrically administered eriodictyol. LC-MS/MS analysis revealed two monoglucuronide metabolites of eriodictyol in liver and intestine microsomes. Recombinant human uridine-5′-diphospho -glucuronosyltransferase (UGT) isoforms and chemical inhibition studies demonstrated that UGT1As (mainly UGT1A1, UGT1A9, UGT1A10) and UGT2B7 were likely the main contributors to eriodictyol glucuronidation. Intragastric administration of eriodictyol, which displayed lower parent and higher metabolite concentrations in the plasma, did not elicit protective effects against APAP hepatotoxicity, when compared to the intraperitoneal injection of eriodictyol. The relative bioavailability of eriodictyol was increased to 216.84% with the coadministration of glycyrrhetinic acid (GA), an inhibitor of UGT1As. Intragastric administration of eriodictyol in combination with GA also induced protective effects against APAP hepatotoxicity. Furthermore, intragastric administration of eriodictyol attenuated APAP hepatotoxicity in heterozygous Ugt1 (Ugt1+/−) mice but not in its wild-type littermates. Thus, UGT1A-mediated metabolic inactivation reduced the protective effect of eriodictyol. Eriodictyol attenuated APAP hepatotoxicity via inhibition of hepatic cytochrome P450 (cyp) 2e1 and cyp3a11 activities; reserve of glutathione (GSH) by improvement of glutathione peroxidase (GSH-Px), glutathione reductase (GR), and glutathione S-transferase (GST) activities; elevation of superoxide dismutase (SOD) activity; and reduction of malondialdehyde (MDA) level. Our findings indicate that parenterally administered eriodictyol may be used to treat APAP-induced hepatotoxicity, and its efficacy can be enhanced by UGT1As down-regulation.
中文翻译:

Eriodictyol,而不是其葡萄糖醛酸苷代谢产物,可减轻对乙酰氨基酚引起的肝毒性。
对乙酰氨基酚(APAP)是最常用的口服镇痛药和退热药之一,但过量用药后可能发生肝毒性,包括肝功能衰竭。治疗APAP肝毒性的治疗选择有限。一种具有抗炎和抗氧化特性的饮食类黄酮Eriodictyol在这里用于确定其对小鼠APAP诱导的肝毒性的保护作用。各种给药途径和药代动力学-药效学(PK-PD)分析可用于确定这些作用。在静脉内和腹膜内观察到保护作用,但在胃内施用的雌黄醇中未观察到保护作用。LC-MS / MS分析显示,肝脏和肠微粒体中有两种二异黄酮代谢物。重组人尿苷5'-二磷酸-葡萄糖醛酸糖基转移酶(UGT)异构体和化学抑制研究表明,UGT1A(主要是UGT1A1,UGT1A9,UGT1A10)和UGT2B7可能是促黄体素葡萄糖醛酸苷化的主要贡献者。与腹膜内注射Eriodictyol相比,胃内注射Eriodictyol在血浆中具有较低的母体含量和较高的代谢产物浓度,并未引起针对APAP肝毒性的保护作用。与UGT1As抑制剂甘草次酸(GA)并用时,雌三醇的相对生物利用度提高到216.84%。胃内联合雌黄醇与GA联合使用也可诱导针对APAP肝毒性的保护作用。此外,Ugt1(Ugt1 +/-)小鼠,但不在其野生型同窝仔中。因此,UGT1A介导的代谢失活降低了雌三醇的保护作用。雌三醇通过抑制肝细胞色素P450(cyp)2e1和cyp3a11活性来减轻APAP的肝毒性;通过改善谷胱甘肽过氧化物酶(GSH-Px),谷胱甘肽还原酶(GR)和谷胱甘肽S-转移酶(GST)活性来保留谷胱甘肽(GSH);超氧化物歧化酶(SOD)活性升高; 并降低丙二醛(MDA)含量。我们的发现表明,肠胃外给药的雌黄醇可用于治疗APAP诱导的肝毒性,其功效可通过UGT1As下调来增强。
更新日期:2017-07-18
中文翻译:

Eriodictyol,而不是其葡萄糖醛酸苷代谢产物,可减轻对乙酰氨基酚引起的肝毒性。
对乙酰氨基酚(APAP)是最常用的口服镇痛药和退热药之一,但过量用药后可能发生肝毒性,包括肝功能衰竭。治疗APAP肝毒性的治疗选择有限。一种具有抗炎和抗氧化特性的饮食类黄酮Eriodictyol在这里用于确定其对小鼠APAP诱导的肝毒性的保护作用。各种给药途径和药代动力学-药效学(PK-PD)分析可用于确定这些作用。在静脉内和腹膜内观察到保护作用,但在胃内施用的雌黄醇中未观察到保护作用。LC-MS / MS分析显示,肝脏和肠微粒体中有两种二异黄酮代谢物。重组人尿苷5'-二磷酸-葡萄糖醛酸糖基转移酶(UGT)异构体和化学抑制研究表明,UGT1A(主要是UGT1A1,UGT1A9,UGT1A10)和UGT2B7可能是促黄体素葡萄糖醛酸苷化的主要贡献者。与腹膜内注射Eriodictyol相比,胃内注射Eriodictyol在血浆中具有较低的母体含量和较高的代谢产物浓度,并未引起针对APAP肝毒性的保护作用。与UGT1As抑制剂甘草次酸(GA)并用时,雌三醇的相对生物利用度提高到216.84%。胃内联合雌黄醇与GA联合使用也可诱导针对APAP肝毒性的保护作用。此外,Ugt1(Ugt1 +/-)小鼠,但不在其野生型同窝仔中。因此,UGT1A介导的代谢失活降低了雌三醇的保护作用。雌三醇通过抑制肝细胞色素P450(cyp)2e1和cyp3a11活性来减轻APAP的肝毒性;通过改善谷胱甘肽过氧化物酶(GSH-Px),谷胱甘肽还原酶(GR)和谷胱甘肽S-转移酶(GST)活性来保留谷胱甘肽(GSH);超氧化物歧化酶(SOD)活性升高; 并降低丙二醛(MDA)含量。我们的发现表明,肠胃外给药的雌黄醇可用于治疗APAP诱导的肝毒性,其功效可通过UGT1As下调来增强。































 京公网安备 11010802027423号
京公网安备 11010802027423号