当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Key Role of the Carboxyl Terminus of Hyaluronan Synthase in Processive Synthesis and Size Control of Hyaluronic Acid Polymers
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-03-08 00:00:00 , DOI: 10.1021/acs.biomac.6b01239 Ji Yang , Fangyu Cheng , Huimin Yu , Junting Wang , Zhigang Guo , Gregory Stephanopoulos 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2017-03-08 00:00:00 , DOI: 10.1021/acs.biomac.6b01239 Ji Yang , Fangyu Cheng , Huimin Yu , Junting Wang , Zhigang Guo , Gregory Stephanopoulos 1
Affiliation
|
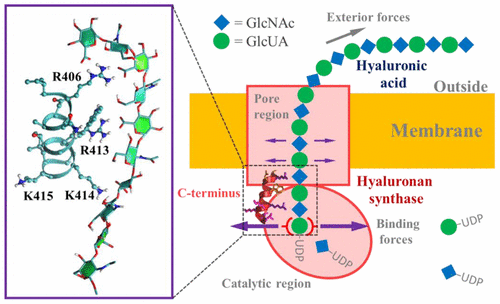
The essential pathophysiological roles of hyaluronic acid (HA) strongly depend on HA binding and HA size. Here we deployed the atomic vision of molecular dynamics (MD) simulation to experimentally investigate the influence of C-terminal mutations of Streptococcus equisimilis hyaluronan synthase (SeHAS) on HA product synthesis in Escherichia coli. R413 was vital for HA production, as the removal or mutation of R413 led to inactivation of SeHAS. MD simulations indicated that R406-R413 constituted an HA-binding pattern that stabilized the HA-SeHAS complex. We further increased HA product size via site-directed mutation of the SeHAS C-terminal residues 414–417 based on the hypothesis that higher binding affinity between the SeHAS C-terminus and HA would lead to larger HA size, underlying the important role of the HA-SeHAS interaction in HA size control. W410A and T412A mutations also completely deactivated SeHAS. Moreover, a catalysis-transformation-translocation model was proposed for the HA synthesis and translocation processes.
中文翻译:

透明质酸合酶羧基末端在透明质酸聚合物的合成和尺寸控制中的关键作用
透明质酸(HA)的基本病理生理作用在很大程度上取决于HA的结合和HA的大小。在这里,我们部署了分子动力学(MD)模拟的原子视觉,以实验研究马链球菌透明质酸合酶(SeHAS)的C端突变对大肠杆菌HA产品合成的影响。R413对HA生产至关重要,因为R413的去除或突变导致SeHAS失活。MD模拟表明,R406-R413构成稳定HA-SeHAS复合物的HA结合模式。基于以下假设,我们通过SeHAS C末端残基414-417的定点突变进一步增加了HA产品的大小,即以下假设:SeHAS C末端与HA之间更高的结合亲和力会导致更大的HA大小,这是潜在的重要作用。 HA大小控制中的HA-SeHAS交互作用。W410A和T412A突变也使SeHAS完全失活。此外,针对HA合成和易位过程,提出了催化-转化-易位模型。
更新日期:2017-03-08
中文翻译:

透明质酸合酶羧基末端在透明质酸聚合物的合成和尺寸控制中的关键作用
透明质酸(HA)的基本病理生理作用在很大程度上取决于HA的结合和HA的大小。在这里,我们部署了分子动力学(MD)模拟的原子视觉,以实验研究马链球菌透明质酸合酶(SeHAS)的C端突变对大肠杆菌HA产品合成的影响。R413对HA生产至关重要,因为R413的去除或突变导致SeHAS失活。MD模拟表明,R406-R413构成稳定HA-SeHAS复合物的HA结合模式。基于以下假设,我们通过SeHAS C末端残基414-417的定点突变进一步增加了HA产品的大小,即以下假设:SeHAS C末端与HA之间更高的结合亲和力会导致更大的HA大小,这是潜在的重要作用。 HA大小控制中的HA-SeHAS交互作用。W410A和T412A突变也使SeHAS完全失活。此外,针对HA合成和易位过程,提出了催化-转化-易位模型。































 京公网安备 11010802027423号
京公网安备 11010802027423号