Cell Chemical Biology ( IF 8.6 ) Pub Date : 2024-05-16 , DOI: 10.1016/j.chembiol.2024.04.007 Xincheng Xu , Zihong Chen , Caroline R. Bartman , Xi Xing , Kellen Olszewski , Joshua D. Rabinowitz
|
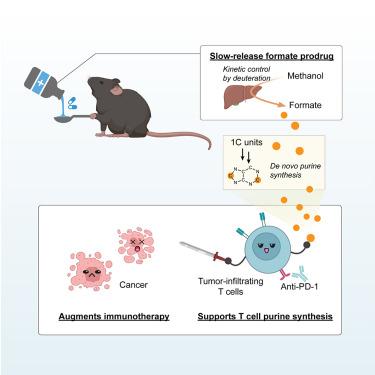
Nucleotides perform important metabolic functions, carrying energy and feeding nucleic acid synthesis. Here, we use isotope tracing-mass spectrometry to quantitate contributions to purine nucleotides from salvage versus de novo synthesis. We further explore the impact of augmenting a key precursor for purine synthesis, one-carbon (1C) units. We show that tumors and tumor-infiltrating T cells (relative to splenic or lymph node T cells) synthesize purines de novo. Shortage of 1C units for T cell purine synthesis is accordingly a potential bottleneck for anti-tumor immunity. Supplementing 1C units by infusing formate drives formate assimilation into purines in tumor-infiltrating T cells. Orally administered methanol functions as a formate pro-drug, with deuteration enabling kinetic control of formate production. Safe doses of methanol raise formate levels and augment anti-PD-1 checkpoint blockade in MC38 tumors, tripling durable regressions. Thus, 1C deficiency can gate antitumor immunity and this metabolic checkpoint can be overcome with pharmacological 1C supplementation.
中文翻译:

一碳单位补充促进肿瘤浸润 T 细胞的嘌呤合成并增强检查点封锁
核苷酸执行重要的代谢功能,携带能量并促进核酸合成。在这里,我们使用同位素示踪质谱法来定量回收与从头合成对嘌呤核苷酸的贡献。我们进一步探讨了增强嘌呤合成的关键前体一碳 (1C) 单元的影响。我们发现肿瘤和肿瘤浸润 T 细胞(相对于脾或淋巴结 T 细胞)从头合成嘌呤。因此,T细胞嘌呤合成的1C单位的短缺是抗肿瘤免疫的潜在瓶颈。通过注入甲酸来补充 1C 单位可促进肿瘤浸润 T 细胞中甲酸同化为嘌呤。口服甲醇可作为甲酸盐前药,通过氘化可对甲酸盐的产生进行动力学控制。安全剂量的甲醇可提高 MC38 肿瘤中的甲酸水平并增强抗 PD-1 检查点阻断,使持久消退效果增加三倍。因此,1C 缺乏可以控制抗肿瘤免疫,并且可以通过药理学补充 1C 来克服这一代谢检查点。
































 京公网安备 11010802027423号
京公网安备 11010802027423号