RSC主编推荐:有机领域精彩文章快览(免费阅读原文)
英国皇家化学会(RSC)是一个超过175年历史的面向全球化学家的非营利会员制机构,旗下拥有44种期刊,其中很多在化学领域有很高影响力。为了进一步帮助广大读者追踪科技前沿热点,X-MOL团队与英国皇家化学会合作,推出英国皇家化学会期刊主编推荐的精彩文章快览,本期文章属“有机领域”,英文点评来自英国皇家化学会期刊的主编。如果大家对我们的解读有更多的补充和点评,欢迎在文末写评论发表您的高见!
Chemical Science (IF: 9.063)

1. A cheap metal for a challenging task: nickel-catalyzed highly diastereo- and enantioselective hydrogenation of tetrasubstituted fluorinated enamides
Chem. Sci., 2018, Advance Article
DOI: 10.1039/ C8SC04002H
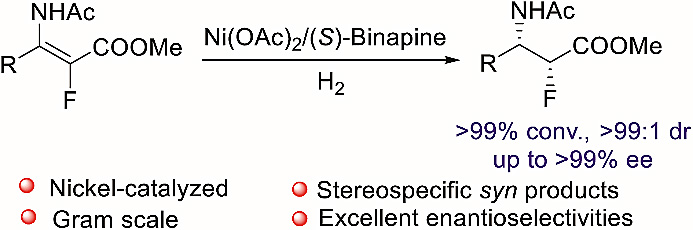
Scientists from Wuhan University have developed a nickel-catalysed asymmetric hydrogenation of tetrasubstituted fluorinated enamides. The protocol gives efficient access to chiral α-fluoro-β-amino esters which are important in organic synthesis and medicinal chemistry.
武汉大学的研究人员发展了一种镍催化四取代氟代烯酰胺的不对称氢化反应。该方法可高效地制备手性α-氟-β-氨基酯,这种结构在有机合成和药物化学中非常重要。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

2. Metal-free, intermolecular carbopyridylation of alkenes via visible-light-induced reductive radical coupling
Chem. Sci., 2018, Advance Article
DOI: 10.1039/C8SC03493A
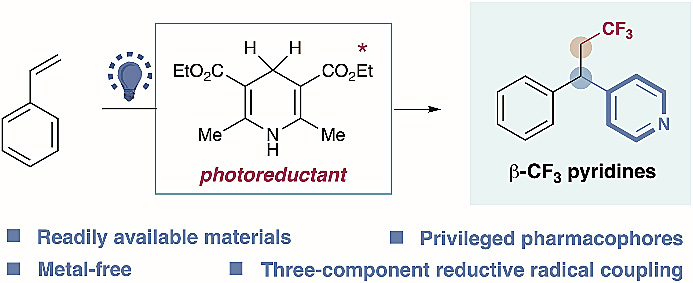
Researchers at Donghua University, Shanghai, have demonstrated an efficient, metal-free strategy for the intermolecular three-component carbopyridylation of styrenes, promoted by Hantzsch ester and visible light, giving access to important β-CF3 pyridines. The authors demonstrate this protocol by functionalising natural product- and drug-based complex molecules.
东华大学的研究人员报道了一种高效、无金属参与的策略,在Hantzsch酯与可见光的促进下,实现了分子间三组分的苯乙烯碳-吡啶双官能化反应,该方法可合成重要的β-CF3吡啶类化合物。作者还将该方法用于官能化基于天然产物和药物的复杂分子。
Open Access(可免费阅读原文)
扫描或长按二维码,识别后直达原文页面,或点此查看原文

Organic Chemistry Frontiers (IF: 5.455)

1. Iridium-catalyzed oxidative Ar–H/Ar–H cross-coupling of primary benzamides with thiophenes
Org. Chem. Front., 2018, 5, 2930-2933
DOI: 10.1039/C8QO00796A
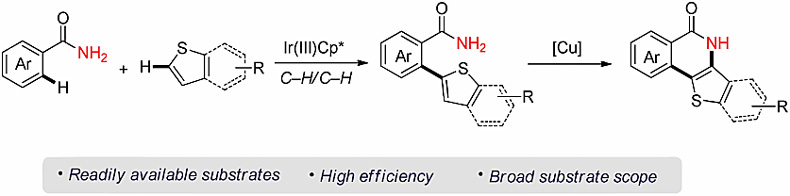
(2-Thienyl)benzamide frameworks are prevalent in pharmaceuticals and advanced materials. Herein, we disclose an iridium-catalyzed oxidative Ar–H/Ar–H cross-coupling reaction of primary benzamides with thiophenes, which provides a rapid pathway to (2-thienyl)benzamide skeletons. Furthermore, the coupled products can be transformed to the corresponding important thiophene-fused 1-isoquinolinones via a copper-catalyzed intramolecular annulation.
(2-噻吩基)苯甲酰胺骨架在药物和先进材料中非常普遍。本文中作者报道了铱催化一级苯甲酰胺与噻吩的氧化Ar–H/Ar–H键交叉偶联反应,由此提供了一种(2-噻吩基)苯甲酰胺骨架快速构建的方法。此外,偶联产物可通过铜催化的分子内增环反应转化为相应的重要的噻吩并1-异喹啉酮。
限时免费阅读原文,登陆后可下载
扫描或长按二维码,识别后直达原文页面,或点此查看原文

如果篇首注明了授权来源,任何转载需获得来源方的许可!如果篇首未特别注明出处,本文版权属于 X-MOL ( x-mol.com ), 未经许可,谢绝转载!































 京公网安备 11010802027423号
京公网安备 11010802027423号