Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Imidazolinium Salts with TIPS Groups for the Palladium-Catalyzed α-Arylation and as Chiral Solvating Agents
Synlett ( IF 1.7 ) Pub Date : 2015-06-18 , DOI: 10.1055/s-0034-1381012 René Wilhelm , Mazhar Gilani , Eduard Rais
Synlett ( IF 1.7 ) Pub Date : 2015-06-18 , DOI: 10.1055/s-0034-1381012 René Wilhelm , Mazhar Gilani , Eduard Rais
|
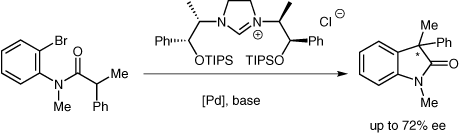
The synthesis of imidazolinium salts from low-cost amino alcohols incorporating different sterically demanding silyl groups is described. The new salts were analyzed and investigated as carbene precursors in a palladium-catalyzed asymmetric intramolecular α-arylation of an amide. The easily accessible carbene precursors gave enantiomeric excesses of up to 72% with nearly quantitative yields. The salts were also successfully applied as chiral solvating agents with the potassium salt of Mosher’s carboxylate and can therefore contribute to the field of chiral recognition.
中文翻译:

具有 TIPS 基团的手性咪唑啉盐,用于钯催化的 α-芳基化和作为手性溶剂化剂
描述了从包含不同空间要求的甲硅烷基的低成本氨基醇合成咪唑啉盐。在钯催化的酰胺的不对称分子内α-芳基化反应中,新盐类作为卡宾前体进行了分析和研究。容易获得的卡宾前体产生高达 72% 的对映体过量,收率几乎是定量的。这些盐还成功地用作 Mosher 羧酸钾盐的手性溶剂化剂,因此可以为手性识别领域做出贡献。
更新日期:2015-06-18
中文翻译:

具有 TIPS 基团的手性咪唑啉盐,用于钯催化的 α-芳基化和作为手性溶剂化剂
描述了从包含不同空间要求的甲硅烷基的低成本氨基醇合成咪唑啉盐。在钯催化的酰胺的不对称分子内α-芳基化反应中,新盐类作为卡宾前体进行了分析和研究。容易获得的卡宾前体产生高达 72% 的对映体过量,收率几乎是定量的。这些盐还成功地用作 Mosher 羧酸钾盐的手性溶剂化剂,因此可以为手性识别领域做出贡献。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号